Definition Of Order Of A Reaction
Muz Play
Mar 29, 2025 · 6 min read
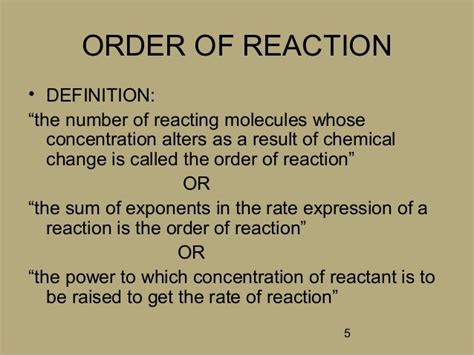
Table of Contents
Defining the Order of a Reaction: A Comprehensive Guide
Chemical kinetics, the study of reaction rates, is crucial for understanding and predicting the behavior of chemical systems. A cornerstone of this field is the concept of the order of a reaction, a quantity that dictates how the rate of a reaction changes with the concentration of its reactants. Understanding the order of a reaction is vital for designing efficient chemical processes, predicting reaction outcomes, and developing sophisticated reaction mechanisms. This comprehensive guide delves deep into the definition of the order of a reaction, exploring various orders, determining reaction order experimentally, and highlighting its significance in chemical kinetics.
What is the Order of a Reaction?
The order of a reaction refers to the power dependence of the reaction rate on the concentration of each reactant. It's a crucial concept because it directly influences how the reaction rate changes as the concentrations of the reactants change. Unlike the stoichiometric coefficients in a balanced chemical equation, the reaction order isn't necessarily an integer and isn't directly derived from the balanced equation. It's determined experimentally. It's important to distinguish between the overall order of a reaction and the order with respect to a specific reactant.
-
Overall order: This represents the sum of the exponents of the concentration terms in the experimentally determined rate law. For example, if the rate law is expressed as
Rate = k[A]²[B], the overall order of the reaction is 2 + 1 = 3 (third-order). -
Order with respect to a specific reactant: This refers to the exponent of the concentration term of a specific reactant in the rate law. In the above example, the reaction is second-order with respect to reactant A and first-order with respect to reactant B.
Different Orders of Reactions: A Detailed Overview
Chemical reactions can exhibit a wide range of orders, from zero-order to complex fractional orders. Let's examine some common types:
1. Zero-Order Reactions
A zero-order reaction is one whose rate is independent of the concentration of the reactants. This unusual behavior often arises when the reaction rate is controlled by a factor other than the reactant concentration, such as the availability of a catalyst or the intensity of light in a photochemical reaction. The rate law for a zero-order reaction is:
Rate = k
where:
Rateis the reaction ratekis the rate constant (with units of concentration/time)
The integrated rate law for a zero-order reaction is:
[A]t = [A]₀ - kt
where:
[A]tis the concentration of reactant A at time t[A]₀is the initial concentration of reactant A
Graphically, a plot of [A]t versus t yields a straight line with a slope of -k.
2. First-Order Reactions
A first-order reaction is one whose rate is directly proportional to the concentration of a single reactant. Many radioactive decay processes and unimolecular reactions fall into this category. The rate law for a first-order reaction is:
Rate = k[A]
where:
Rateis the reaction ratekis the rate constant (with units of 1/time)[A]is the concentration of reactant A
The integrated rate law is:
ln[A]t = ln[A]₀ - kt
or, equivalently:
[A]t = [A]₀e^(-kt)
A plot of ln[A]t versus t gives a straight line with a slope of -k. The half-life (t<sub>1/2</sub>) of a first-order reaction, the time it takes for the reactant concentration to halve, is independent of the initial concentration and is given by:
t<sub>1/2</sub> = 0.693/k
3. Second-Order Reactions
A second-order reaction can involve either two molecules of the same reactant or one molecule each of two different reactants. The rate depends on the square of the concentration of one reactant or the product of the concentrations of two reactants.
- Second-order with respect to one reactant: The rate law is:
Rate = k[A]²
The integrated rate law is:
1/[A]t = 1/[A]₀ + kt
A plot of 1/[A]t versus t yields a straight line with slope k. The half-life is dependent on the initial concentration:
t<sub>1/2</sub> = 1/(k[A]₀)
- Second-order with respect to two reactants: The rate law is:
Rate = k[A][B]
The integrated rate law is more complex and depends on whether the initial concentrations of A and B are equal or not.
4. Higher-Order and Fractional-Order Reactions
Reactions with orders higher than second-order are less common but can occur. Fractional orders indicate that the reaction mechanism is more complex, often involving multiple steps. Determining the rate law experimentally is crucial for these reactions.
Determining the Order of a Reaction: Experimental Methods
The order of a reaction cannot be determined simply by looking at the stoichiometric equation. Experimental methods are essential. Common techniques include:
-
Method of Initial Rates: This method involves measuring the initial rate of the reaction at different initial concentrations of reactants. By comparing the changes in initial rates with changes in concentration, the order with respect to each reactant can be determined.
-
Graphical Method: Plotting the concentration of reactant(s) versus time in different ways (e.g., [A] vs t, ln[A] vs t, 1/[A] vs t) allows determination of the reaction order based on the linearity of the resulting graphs.
-
Half-life Method: Measuring the half-life of the reaction at different initial concentrations can reveal the reaction order. For first-order reactions, the half-life is independent of the initial concentration, while for second-order reactions, the half-life is inversely proportional to the initial concentration.
Significance of Reaction Order
Understanding the order of a reaction has numerous practical implications:
-
Reaction Rate Prediction: Knowing the rate law allows us to predict the rate of the reaction under various conditions, which is crucial for process optimization and control.
-
Mechanism Elucidation: The reaction order provides valuable insights into the reaction mechanism. For example, a simple, elementary reaction will have a rate law directly related to its stoichiometry, while complex reactions involving multiple steps will often have non-integer or complex rate laws.
-
Process Design: In industrial applications, knowledge of the reaction order is vital for designing efficient reactors and controlling reaction conditions to maximize yield and minimize unwanted side reactions.
-
Pharmacokinetics and Toxicology: In pharmacology and toxicology, the order of metabolic reactions plays a significant role in determining drug efficacy, dosage regimens, and the body’s response to toxic substances.
Advanced Concepts and Considerations
-
Pseudo-order reactions: In reactions with multiple reactants, if one reactant is present in significant excess compared to others, its concentration remains essentially constant during the reaction. This simplifies the rate law, making it appear to be of lower order than the overall order.
-
Complex reaction mechanisms: Many reactions involve multiple elementary steps. The overall reaction order may not be directly related to the stoichiometry of the overall reaction, requiring detailed kinetic analysis to decipher.
-
Temperature dependence: The rate constant (k) is highly sensitive to temperature, typically following the Arrhenius equation. This temperature dependence is significant for understanding reaction dynamics under varying conditions.
Conclusion
The order of a reaction is a fundamental concept in chemical kinetics, directly influencing reaction rates and providing valuable insights into reaction mechanisms. Determining the reaction order experimentally, using methods like the initial rates method or graphical analysis, is crucial for predicting reaction behavior and optimizing chemical processes. A thorough understanding of reaction orders is essential for scientists and engineers across various fields, from chemistry and chemical engineering to pharmacology and environmental science. The concepts discussed here provide a comprehensive foundation for further exploration of this important topic in chemical kinetics.
Latest Posts
Latest Posts
-
Principle Of Conservation Of Angular Momentum
Mar 31, 2025
-
Thesis Statement Of A Narrative Essay
Mar 31, 2025
-
Ionic Compounds Dissociate In Water Into
Mar 31, 2025
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
-
Is Ph A Chemical Or Physical Property
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Definition Of Order Of A Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
