What Elements Are Most To Become Anions
Muz Play
Mar 29, 2025 · 5 min read
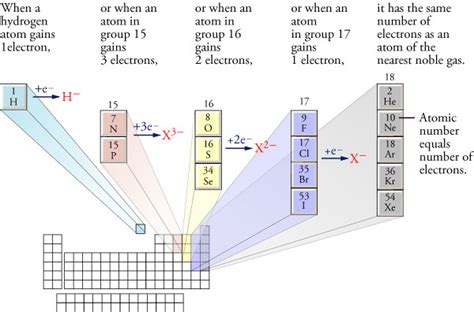
Table of Contents
What Elements Are Most Likely to Become Anions?
Understanding the formation of anions—negatively charged ions—is crucial to comprehending chemical bonding and reactivity. This article delves into the factors determining an element's propensity to gain electrons and become an anion, exploring the periodic trends and exceptions that govern this fundamental process.
The Role of Electronegativity
The most significant factor influencing an element's likelihood of forming an anion is its electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Elements with high electronegativity are more likely to gain electrons and form anions because they strongly attract electrons from other atoms.
Periodic Trends in Electronegativity
Electronegativity generally increases across a period (from left to right) on the periodic table and decreases down a group (from top to bottom). This means that elements in the upper right-hand corner of the periodic table, excluding the noble gases, tend to exhibit the highest electronegativity and therefore the greatest tendency to form anions.
-
Across a period: As you move across a period, the effective nuclear charge increases, pulling the valence electrons closer to the nucleus and increasing their attraction to incoming electrons.
-
Down a group: As you move down a group, the atomic radius increases, meaning the valence electrons are farther from the nucleus and experience less attraction. Shielding by inner electrons also becomes more significant, further reducing the effective nuclear charge.
The Importance of Electron Configuration
The electron configuration of an element dictates its reactivity and tendency to form anions. Elements are most stable when their outermost electron shell (valence shell) is full. This is often achieved by gaining electrons to reach a noble gas configuration (eight electrons in the outermost shell, the octet rule).
Achieving a Stable Octet
Elements readily gain electrons to complete their valence shell, especially if they are only one or two electrons short of a full octet. This is why elements in Groups 16 (chalcogens) and 17 (halogens) are particularly prone to forming anions.
-
Group 16 (Chalcogens): Oxygen, sulfur, selenium, and tellurium all have six valence electrons. By gaining two electrons, they achieve a stable octet and form anions with a -2 charge (e.g., O²⁻, S²⁻, Se²⁻, Te²⁻).
-
Group 17 (Halogens): Fluorine, chlorine, bromine, and iodine have seven valence electrons. They readily gain one electron to complete their octet and form anions with a -1 charge (e.g., F⁻, Cl⁻, Br⁻, I⁻).
Exceptions to the Rules
While electronegativity and electron configuration are the primary factors, there are exceptions to the general trends:
-
Transition Metals: Transition metals often exhibit variable oxidation states, meaning they can lose or gain varying numbers of electrons. While they can form anions, it's less common than for elements in Groups 16 and 17. Their multiple oxidation states result from the involvement of d-electrons in bonding, leading to complex behavior.
-
Post-Transition Metals: Elements like lead and tin can form both cations and anions, depending on the specific chemical environment. This is due to their relatively low electronegativity and the possibility of engaging both s and p electrons in bonding.
-
Large Anions: The formation of highly charged anions (e.g., -3, -4) becomes less favorable with increasing atomic size due to the increasing electron-electron repulsion. This effect is particularly prominent for larger elements in the lower periods of the periodic table.
-
Noble Gases: Noble gases have a complete octet and are very unreactive. They rarely form anions, demonstrating the exceptional stability of a filled valence shell.
Factors Affecting Anion Formation: Beyond Electronegativity
Beyond electronegativity and electron configuration, other factors influence anion formation:
-
Ionization Energy: This is the energy required to remove an electron from a neutral atom. Elements with low ionization energies are less likely to form anions because it requires less energy to lose an electron than to gain one.
-
Lattice Energy: This is the energy released when ions come together to form a crystal lattice. High lattice energy favors the formation of ionic compounds and therefore the formation of anions. The strong electrostatic attraction between the cation and anion in the crystal lattice stabilizes the ionic compound.
-
Size and Charge: The size and charge of the cation involved in the ionic compound also affect the anion formation. A smaller, highly charged cation will exert a stronger electrostatic attraction on the anion, making anion formation more favorable.
Examples of Common Anions
Numerous elements readily form anions. Some prominent examples include:
-
Halide ions: Fluoride (F⁻), chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻) are ubiquitous in various chemical compounds.
-
Oxide ion: The oxide ion (O²⁻) is a fundamental component of many minerals and inorganic compounds.
-
Sulfide ion: The sulfide ion (S²⁻) is found in metal sulfides, which have significant geological and industrial applications.
-
Nitride ion: The nitride ion (N³⁻) is less common than halide and oxide ions but still occurs in certain compounds.
-
Phosphate ion: The phosphate ion (PO₄³⁻) is a crucial component in biological systems and fertilizers.
The Significance of Anion Formation in Chemistry
The formation of anions is fundamental to many chemical processes and phenomena. Understanding the factors governing anion formation is critical for:
-
Predicting chemical reactivity: Knowing which elements are likely to form anions helps predict how they will react with other elements.
-
Designing new materials: The properties of materials are often dictated by the ions that make up their structure. Understanding anion formation is therefore crucial in materials science.
-
Understanding biological processes: Many biological processes rely on the interaction of ions, including anions. Understanding anion formation is essential in biochemistry and related fields.
-
Environmental chemistry: Anions play a significant role in various environmental processes, such as water chemistry and soil nutrient cycling.
Conclusion
Anion formation is a complex phenomenon governed by several interconnected factors. While electronegativity and the drive to achieve a stable electron configuration are the most significant drivers, other factors like ionization energy, lattice energy, and the size and charge of participating ions also play important roles. Understanding these principles is paramount to comprehending chemical bonding, predicting chemical reactivity, and developing new materials and technologies. By examining the periodic trends and the exceptions, we can better predict which elements will readily gain electrons and form negatively charged ions, leading to a deeper understanding of the fundamental principles of chemistry.
Latest Posts
Latest Posts
-
Principle Of Conservation Of Angular Momentum
Mar 31, 2025
-
Thesis Statement Of A Narrative Essay
Mar 31, 2025
-
Ionic Compounds Dissociate In Water Into
Mar 31, 2025
-
The Ends Of Long Bones Are Called The
Mar 31, 2025
-
Is Ph A Chemical Or Physical Property
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Elements Are Most To Become Anions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
