Can A Non-polar Molecule Contain Polar Bonds
Muz Play
Mar 18, 2025 · 5 min read
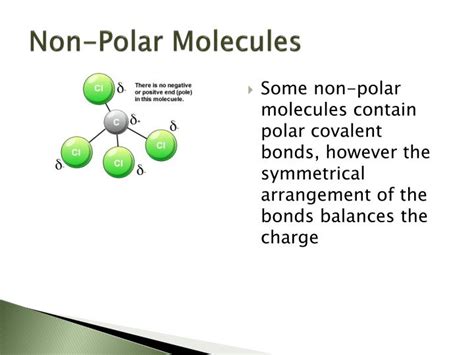
Table of Contents
Can a Non-Polar Molecule Contain Polar Bonds? A Deep Dive into Molecular Polarity
The concept of molecular polarity is fundamental to understanding chemical behavior. While the presence of polar bonds can contribute to overall molecular polarity, it's crucial to remember that a molecule's polarity is a consequence of both the polarity of its individual bonds and its overall geometry. This means that a molecule can indeed possess polar bonds yet remain non-polar as a whole. This article will explore this fascinating contradiction in detail, examining the factors that determine molecular polarity and providing clear examples to illustrate the concept.
Understanding Polar Bonds
A polar bond arises when there's a significant difference in electronegativity between two atoms participating in a covalent bond. Electronegativity, a measure of an atom's ability to attract shared electrons in a chemical bond, increases across a period and decreases down a group in the periodic table. When one atom is significantly more electronegative than the other, it pulls the shared electrons closer to itself, creating a dipole moment. This dipole moment is represented by a vector pointing from the less electronegative atom to the more electronegative atom. The greater the electronegativity difference, the larger the dipole moment and the more polar the bond.
Examples of Polar Bonds
- H-Cl (Hydrogen Chloride): Chlorine is significantly more electronegative than hydrogen, resulting in a highly polar bond.
- O-H (Hydroxyl group): Oxygen is more electronegative than hydrogen, leading to a polar O-H bond, common in alcohols and water.
- C=O (Carbonyl group): Oxygen is more electronegative than carbon, resulting in a polar C=O bond found in ketones, aldehydes, and carboxylic acids.
Molecular Geometry and the Overall Dipole Moment
The individual bond dipoles within a molecule don't tell the whole story. The three-dimensional arrangement of atoms (molecular geometry) plays a crucial role in determining whether the molecule is polar or non-polar. This is because individual bond dipoles can cancel each other out, resulting in a net dipole moment of zero.
Vector Addition of Bond Dipoles
Imagine each bond dipole as a vector. The overall molecular dipole moment is the vector sum of all individual bond dipoles. If these vectors cancel each other out, the molecule is non-polar, even if it contains polar bonds. This cancellation is dependent on both the magnitude of the individual bond dipoles and the angles between them.
Symmetrical Molecules: A Path to Non-Polarity
Symmetrical molecules often exhibit this cancellation effect. If the molecule's geometry is such that the individual bond dipoles are equal in magnitude and oriented symmetrically, they will cancel out. This results in a net dipole moment of zero, rendering the molecule non-polar.
Examples of Non-Polar Molecules with Polar Bonds
Several common molecules showcase the phenomenon of polar bonds within a non-polar molecule. Let's examine some key examples:
1. Carbon Dioxide (CO₂)
Carbon dioxide is a linear molecule with two C=O double bonds. Each C=O bond is polar, with oxygen being more electronegative than carbon. However, because the molecule is linear, the two bond dipoles are equal in magnitude and point in opposite directions. Therefore, they cancel each other out, resulting in a non-polar molecule.
Visual Representation:
Imagine two vectors pointing away from the central carbon atom, one representing each C=O bond dipole. Since they are equal and opposite, their sum is zero.
2. Methane (CH₄)
Methane has four C-H bonds. Although the C-H bond is slightly polar (carbon is slightly more electronegative than hydrogen), the tetrahedral geometry of methane means the four bond dipoles are equal in magnitude and point towards the corners of a tetrahedron. These dipoles perfectly cancel each other out, leading to a non-polar molecule.
Visual Representation:
Picture four vectors pointing from the central carbon atom towards the vertices of a tetrahedron. The symmetry ensures complete cancellation.
3. Boron Trifluoride (BF₃)
Boron trifluoride has three B-F bonds arranged in a trigonal planar geometry. Each B-F bond is polar because fluorine is much more electronegative than boron. However, due to the symmetrical planar arrangement, the three bond dipoles cancel each other out, leading to a non-polar molecule.
Visual Representation:
Imagine three vectors pointing from the central boron atom at 120° angles. Their equal magnitudes and symmetrical arrangement ensure a net dipole moment of zero.
4. Carbon Tetrachloride (CCl₄)
Similar to methane, carbon tetrachloride exhibits a tetrahedral geometry with four C-Cl bonds. While the C-Cl bond is polar (chlorine is more electronegative than carbon), the symmetrical arrangement of these polar bonds causes their dipole moments to cancel out, resulting in a non-polar molecule.
Factors Influencing Molecular Polarity: A Recap
The polarity of a molecule hinges on two key factors:
- Bond Polarity: Determined by the electronegativity difference between bonded atoms. A larger difference leads to a more polar bond.
- Molecular Geometry: The three-dimensional arrangement of atoms significantly influences the cancellation or reinforcement of individual bond dipoles. Symmetrical geometries often lead to non-polar molecules, even with polar bonds.
Implications of Molecular Polarity
Understanding molecular polarity is vital because it significantly impacts a molecule's physical and chemical properties:
- Solubility: Polar molecules generally dissolve in polar solvents (like water), while non-polar molecules dissolve in non-polar solvents (like oil). This is the basis of the "like dissolves like" principle.
- Boiling and Melting Points: Polar molecules tend to have higher boiling and melting points than non-polar molecules due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding).
- Reactivity: Polarity influences a molecule's reactivity, particularly in reactions involving polar reactants. Polar molecules are more likely to participate in reactions with other polar molecules.
Conclusion: A nuanced understanding of molecular polarity
The relationship between polar bonds and overall molecular polarity is not straightforward. While the presence of polar bonds can contribute to overall polarity, the molecule's geometry plays a decisive role. Symmetrical molecular geometries can lead to the cancellation of individual bond dipoles, resulting in a non-polar molecule despite the presence of polar bonds. This understanding is critical in predicting the physical and chemical properties of various compounds and for comprehending their behavior in different environments. Understanding this crucial concept helps to bridge the gap between individual bond properties and macroscopic molecular behavior. The examples discussed highlight the importance of considering both bond polarity and molecular geometry for accurate predictions of molecular polarity. Therefore, a thorough appreciation of this nuanced concept is essential for mastering chemistry.
Latest Posts
Latest Posts
-
Is Water A Mixture Or Pure Substance
Mar 18, 2025
-
Which Base Is Not Found In Rna
Mar 18, 2025
-
So Long To Pinky Here Comes The Thumb
Mar 18, 2025
-
When Two Amino Acids Are Joined Together
Mar 18, 2025
-
What Is The Difference Between Chemical Reaction And Nuclear Reaction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Can A Non-polar Molecule Contain Polar Bonds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
