Chemical Equilibrium Le Chatelier's Principle Lab
Muz Play
Mar 16, 2025 · 6 min read
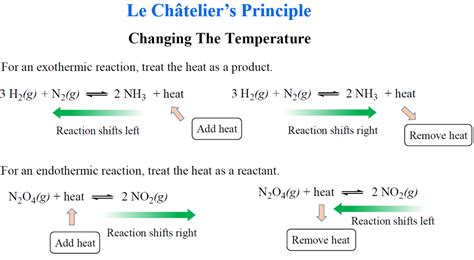
Table of Contents
Chemical Equilibrium and Le Chatelier's Principle: A Comprehensive Lab Exploration
Chemical equilibrium, a cornerstone of chemistry, describes the dynamic state where the rates of the forward and reverse reactions are equal, resulting in no net change in the concentrations of reactants and products. Understanding this dynamic balance is crucial for numerous applications, from industrial chemical processes to biological systems. Le Chatelier's principle further elucidates this equilibrium, stating that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. This lab report delves into a comprehensive exploration of chemical equilibrium and Le Chatelier's principle through a series of experiments.
Understanding Chemical Equilibrium
Before diving into the experimental procedures, it's crucial to grasp the fundamental concepts of chemical equilibrium. Consider a reversible reaction:
aA + bB ⇌ cC + dD
Where:
- a, b, c, and d represent the stoichiometric coefficients
- A and B are reactants
- C and D are products
At equilibrium, the forward reaction rate (A and B forming C and D) equals the reverse reaction rate (C and D forming A and B). This doesn't imply that the concentrations of reactants and products are equal; rather, it signifies a dynamic balance where the rates are equal. The equilibrium constant, K<sub>eq</sub>, quantifies this equilibrium:
K<sub>eq</sub> = [C]<sup>c</sup>[D]<sup>d</sup> / [A]<sup>a</sup>[B]<sup>b</sup>
Where the bracketed terms represent the equilibrium concentrations. A large K<sub>eq</sub> indicates that the equilibrium favors the products, while a small K<sub>eq</sub> signifies that the equilibrium favors the reactants.
Factors Affecting Equilibrium
Several factors can disrupt the equilibrium, causing a shift in the reaction to re-establish a new equilibrium state. These are the key aspects addressed by Le Chatelier's principle:
-
Concentration Changes: Adding more reactants shifts the equilibrium to the right (favoring product formation), while adding more products shifts it to the left (favoring reactant formation). Removing reactants or products has the opposite effect.
-
Temperature Changes: The effect of temperature changes depends on whether the reaction is exothermic (releases heat) or endothermic (absorbs heat). Increasing the temperature of an endothermic reaction shifts the equilibrium to the right, while increasing the temperature of an exothermic reaction shifts it to the left. The opposite is true for decreasing temperature.
-
Pressure Changes: Changes in pressure significantly affect reactions involving gases. Increasing the pressure shifts the equilibrium towards the side with fewer gas molecules, while decreasing the pressure shifts it towards the side with more gas molecules. This is because pressure is directly related to the concentration of gases.
Le Chatelier's Principle: Experimental Verification
This section details the experimental procedures used to verify Le Chatelier's principle. Several different equilibrium systems were investigated to demonstrate the effects of concentration, temperature, and pressure changes.
Experiment 1: The Effect of Concentration Changes on the Iron(III) Thiocyanate Equilibrium
This experiment utilizes the equilibrium between iron(III) ions (Fe<sup>3+</sup>), thiocyanate ions (SCN<sup>-</sup>), and the iron(III) thiocyanate complex ion ([Fe(SCN)]<sup>2+</sup>):
Fe<sup>3+</sup>(aq) + SCN<sup>-</sup>(aq) ⇌ [Fe(SCN)]<sup>2+</sup>(aq)
The solution is initially a deep red color due to the formation of the complex ion. By adding different reagents, we observe shifts in equilibrium based on Le Chatelier's principle.
Procedure:
-
Prepare a solution of iron(III) nitrate and potassium thiocyanate. The deep red color indicates the presence of the [Fe(SCN)]<sup>2+</sup> complex.
-
Stress 1: Adding Fe<sup>3+</sup>: Add a few drops of iron(III) nitrate solution. Observe the change in color intensity. The increased concentration of Fe<sup>3+</sup> drives the equilibrium to the right, increasing the [Fe(SCN)]<sup>2+</sup> concentration and deepening the red color.
-
Stress 2: Adding SCN<sup>-</sup>: Add a few drops of potassium thiocyanate solution. Observe the change in color intensity. Similar to stress 1, the increased concentration of SCN<sup>-</sup> shifts the equilibrium right, deepening the color.
-
Stress 3: Adding a Precipitating Agent for Fe<sup>3+</sup>: Add a few drops of sodium fluoride (NaF). Fluoride ions react with Fe<sup>3+</sup> to form a stable complex, effectively reducing the Fe<sup>3+</sup> concentration. The equilibrium shifts to the left, causing the color to fade.
-
Stress 4: Adding a Precipitating Agent for SCN<sup>-</sup>: Add a few drops of silver nitrate (AgNO<sub>3</sub>). Silver ions react with SCN<sup>-</sup> to form a precipitate (AgSCN), reducing the SCN<sup>-</sup> concentration. The equilibrium shifts left, causing the color to fade.
Experiment 2: The Effect of Temperature Changes on the Cobalt(II) Chloride Equilibrium
This experiment involves the equilibrium between the pink hexaaquacobalt(II) ion ([Co(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup>) and the blue tetrachlorocobaltate(II) ion ([CoCl<sub>4</sub>]<sup>2-</sup>):
[Co(H<sub>2</sub>O)<sub>6</sub>]<sup>2+</sup>(aq) + 4Cl<sup>-</sup>(aq) ⇌ [CoCl<sub>4</sub>]<sup>2-</sup>(aq) + 6H<sub>2</sub>O(l) (Heat is absorbed in the forward direction)
The color change reflects the shift in equilibrium due to temperature variations.
Procedure:
-
Prepare a solution of cobalt(II) chloride. The solution will be pink at room temperature.
-
Stress 1: Increasing Temperature: Heat the solution gently in a hot water bath. Observe the color change. The endothermic nature of the forward reaction causes the equilibrium to shift to the right as temperature increases, resulting in a blue color.
-
Stress 2: Decreasing Temperature: Cool the solution in an ice bath. Observe the color change. As the temperature decreases, the equilibrium shifts to the left, and the solution returns to its pink color.
Experiment 3: The Effect of Pressure Changes on a Gaseous Equilibrium (Optional)
This experiment would typically involve a gaseous equilibrium, such as the Haber-Bosch process for ammonia synthesis:
N<sub>2</sub>(g) + 3H<sub>2</sub>(g) ⇌ 2NH<sub>3</sub>(g)
Due to the complexity of manipulating gaseous pressures in a standard laboratory setting, this experiment might be simulated using data analysis or theoretical calculations to demonstrate Le Chatelier's principle.
Data Analysis and Interpretation
The experimental data collected, including color intensity changes, should be carefully analyzed and interpreted in light of Le Chatelier's principle. Quantitative measurements, such as spectrophotometric readings to determine the absorbance of the colored solutions, can enhance the accuracy of the analysis. Graphs depicting the effect of concentration or temperature on the equilibrium position can also visually reinforce the observed trends.
Conclusion
This laboratory investigation effectively demonstrates the principles of chemical equilibrium and Le Chatelier's principle. The experiments highlight how changes in concentration, temperature, and pressure (in appropriate systems) influence the position of equilibrium, causing a shift to relieve the applied stress. The observed color changes in the iron(III) thiocyanate and cobalt(II) chloride systems provide clear visual evidence of these equilibrium shifts. The data analysis strengthens the conclusions drawn, offering a quantitative perspective to the qualitative observations. This thorough understanding of equilibrium is vital for various applications in chemistry and beyond. Further experiments could explore different equilibrium systems or incorporate more sophisticated analytical techniques for more robust results.
Latest Posts
Latest Posts
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Chemical Equilibrium Le Chatelier's Principle Lab . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
