Chemical Formula Of Nitrogen And Hydrogen
Muz Play
Mar 28, 2025 · 6 min read
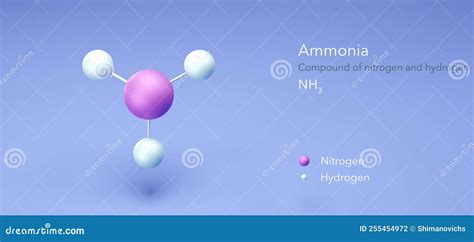
Table of Contents
Delving Deep into the Chemical Formulas of Nitrogen and Hydrogen: A Comprehensive Guide
Nitrogen and hydrogen, two seemingly simple elements, play pivotal roles in the universe and our daily lives. Understanding their chemical formulas, along with their properties and interactions, is fundamental to grasping numerous scientific concepts, from basic chemistry to advanced atmospheric studies and industrial processes. This in-depth article explores the chemical formulas of nitrogen and hydrogen, delving into their individual characteristics and the significant compounds they form when combined.
Understanding Nitrogen: Formula and Properties
Nitrogen (N), the seventh element on the periodic table, exists primarily as a diatomic gas (N<sub>2</sub>) in its elemental form. This means that two nitrogen atoms are strongly bonded together covalently to form a stable molecule. This diatomic nature significantly impacts its reactivity.
The Significance of the N<sub>2</sub> Formula
The chemical formula N<sub>2</sub> is crucial because it highlights the triple bond between the two nitrogen atoms. This triple bond (N≡N) is exceptionally strong, requiring a substantial amount of energy to break. This high bond energy is responsible for nitrogen's relative inertness at room temperature, making it a crucial component of our atmosphere. Without this strong triple bond, life as we know it would be dramatically different.
Key Properties of Nitrogen
- Gas at room temperature: Nitrogen's inertness stems largely from the strong triple bond in N<sub>2</sub>.
- Colorless, odorless, and tasteless: These characteristics contribute to its unobtrusive presence in the air.
- Slightly soluble in water: This low solubility affects its biological availability and its role in aquatic environments.
- Essential nutrient: Despite its inertness, nitrogen is crucial for life. Plants absorb nitrogen in various forms (nitrates, nitrites, ammonia) to synthesize essential amino acids and proteins.
- Industrial applications: Nitrogen is used extensively in various industries, including food preservation (as an inert atmosphere), ammonia production (Haber-Bosch process), and the creation of specialized alloys.
Hydrogen: Formula and its Unique Characteristics
Hydrogen (H), the lightest element, also exists primarily as a diatomic gas (H<sub>2</sub>) under standard conditions. Unlike nitrogen, its bond is a single covalent bond (H-H), making it significantly more reactive.
The H<sub>2</sub> Formula: Simplicity and Reactivity
The simple formula H<sub>2</sub> represents a molecule of hydrogen gas. The single covalent bond between the two hydrogen atoms is relatively weak compared to nitrogen's triple bond. This weaker bond translates to higher reactivity. Hydrogen readily forms bonds with numerous other elements, participating in a wide variety of chemical reactions.
Defining Properties of Hydrogen
- Colorless, odorless, and tasteless gas: Similar to nitrogen, hydrogen's sensory neutrality often masks its presence.
- Highly flammable: The combustion of hydrogen produces only water, making it a potential clean energy source.
- Low density: Hydrogen is the least dense element, making it ideal for applications requiring lightweight materials.
- Excellent reducing agent: Hydrogen readily donates electrons in chemical reactions, reducing other substances. This property is widely used in industrial processes.
- Abundant in the universe: Hydrogen is the most abundant element in the universe, forming a major component of stars and interstellar matter.
The Crucial Role of Nitrogen and Hydrogen in the Haber-Bosch Process
One of the most significant industrial applications showcasing the interaction of nitrogen and hydrogen is the Haber-Bosch process. This method revolutionized agriculture by producing ammonia (NH<sub>3</sub>) on an industrial scale.
The Chemistry of Ammonia Production
The Haber-Bosch process involves the reaction of nitrogen gas (N<sub>2</sub>) and hydrogen gas (H<sub>2</sub>) under high pressure (typically 200-400 atm) and high temperature (400-500°C) in the presence of an iron catalyst. The balanced chemical equation is:
N<sub>2</sub>(g) + 3H<sub>2</sub>(g) ⇌ 2NH<sub>3</sub>(g)
This reaction is reversible and exothermic (releases heat). The conditions used in the Haber-Bosch process are carefully optimized to maximize ammonia yield. The high pressure favors the formation of ammonia, as it reduces the number of gas molecules. The catalyst speeds up the reaction rate, making the process economically viable.
Ammonia's Impact on Agriculture and Beyond
Ammonia, produced through the Haber-Bosch process, is crucial for the manufacture of fertilizers. Nitrogen is a key nutrient for plant growth, and ammonia serves as a readily available source of nitrogen for plants. The widespread availability of nitrogen fertilizers, a direct result of the Haber-Bosch process, has drastically increased agricultural yields globally, supporting a significant portion of the world's population. Ammonia also has applications in various other industries, including the production of explosives, pharmaceuticals, and cleaning agents.
Beyond Ammonia: Other Important Compounds
While ammonia is the most prominent compound formed by nitrogen and hydrogen, other important compounds exist, each with its own unique properties and applications.
Hydrazine (N<sub>2</sub>H<sub>4</sub>)
Hydrazine is a highly reactive compound used as a rocket propellant, owing to its high energy density. Its chemical formula indicates a nitrogen-nitrogen single bond and two nitrogen-hydrogen bonds. Its toxicity mandates careful handling.
Hydroxylamine (NH<sub>2</sub>OH)
Hydroxylamine is a weaker reducing agent compared to hydrazine and finds application as a reagent in organic chemistry. Its formula showcases a nitrogen atom bonded to two hydrogens and a hydroxyl group.
Ammonium Salts (NH<sub>4</sub><sup>+</sup>)
Ammonium ions (NH<sub>4</sub><sup>+</sup>) are formed when ammonia accepts a proton (H<sup>+</sup>). These ions form various salts with different anions (e.g., ammonium chloride, NH<sub>4</sub>Cl; ammonium nitrate, NH<sub>4</sub>NO<sub>3</sub>), each with specific properties and uses. Ammonium nitrate is a vital component of many fertilizers.
Environmental Considerations
While the Haber-Bosch process has significantly enhanced food production, it also carries environmental consequences. The production of ammonia requires vast amounts of energy, contributing to greenhouse gas emissions. The widespread use of nitrogen fertilizers can lead to eutrophication in water bodies, harming aquatic ecosystems. Furthermore, the production and use of other nitrogen-hydrogen compounds can have environmental implications, necessitating sustainable practices and responsible usage.
Future Directions and Research
Research continues to focus on improving the efficiency and sustainability of ammonia production and exploring alternative methods for nitrogen fixation. Scientists are actively investigating novel catalysts and reaction conditions to reduce energy consumption and greenhouse gas emissions associated with the Haber-Bosch process. Exploring alternative nitrogen sources for fertilizers and developing methods for minimizing environmental impact are essential for sustainable development. Research into the development of new nitrogen-hydrogen compounds with specific applications in materials science, energy storage, and medicine also promises exciting advancements in various fields.
Conclusion: The Ubiquitous Nature of Nitrogen and Hydrogen
The chemical formulas of nitrogen (N<sub>2</sub>) and hydrogen (H<sub>2</sub>) are deceptively simple, yet they underpin a vast array of natural processes and industrial applications. Their unique properties and interactions, particularly the formation of ammonia through the Haber-Bosch process, have profoundly impacted human civilization. Understanding these formulas and their implications is crucial for appreciating the interconnectedness of chemistry, biology, and environmental science. As we move forward, addressing the environmental challenges associated with their use and exploring new avenues of application remain vital for ensuring a sustainable future.
Latest Posts
Latest Posts
-
Electric Field Of A Charged Disk
Mar 31, 2025
-
5 Blind Man And The Elephant
Mar 31, 2025
-
Which Statement Describes The Citric Acid Cycle
Mar 31, 2025
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Chemical Formula Of Nitrogen And Hydrogen . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
