Chemical Reaction Of Metals With Bases
Muz Play
Mar 23, 2025 · 5 min read
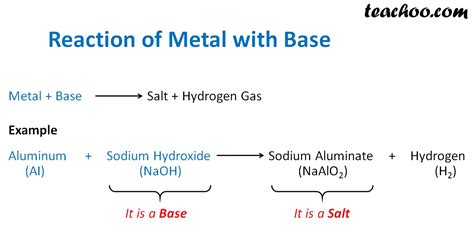
Table of Contents
Chemical Reactions of Metals with Bases: A Deep Dive
The interaction between metals and bases, while less frequently discussed than reactions with acids, is a significant area of chemistry with diverse applications. This detailed exploration will delve into the intricacies of these reactions, covering various aspects, including the types of metals involved, the reaction mechanisms, influencing factors, and practical applications.
Understanding the Fundamentals
Before diving into the specifics, let's establish a foundational understanding. Bases, also known as alkalis, are substances that, in aqueous solutions, release hydroxide ions (OH⁻). These ions are the key players in the reactions with metals. Metals, on the other hand, are elements characterized by their tendency to lose electrons and form positive ions (cations). This electron donation is crucial to the reaction process.
The reaction between a metal and a base is fundamentally a redox reaction, involving both reduction and oxidation. The metal undergoes oxidation (loss of electrons), while a component of the base undergoes reduction (gain of electrons). The specific nature of the reduction process varies depending on the base and the reaction conditions.
Types of Metals Reacting with Bases
Not all metals react readily with bases. The reactivity is largely dictated by the metal's position in the electrochemical series. Generally, metals that are more reactive than hydrogen, and specifically those with a relatively low ionization energy, are more prone to reacting with bases. These typically include:
1. Amphoteric Metals:
These metals exhibit a unique property – they can react with both acids and bases. Aluminum (Al) and Zinc (Zn) are prime examples. Their reactions with bases produce a salt and hydrogen gas. For example:
- 2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g) (Reaction of Aluminum with Sodium Hydroxide)
- Zn(s) + 2NaOH(aq) → Na₂ZnO₂(aq) + H₂(g) (Reaction of Zinc with Sodium Hydroxide)
Note that the products in these reactions often involve complex ions, such as the tetrahydroxoaluminate ion [Al(OH)₄]⁻ for aluminum.
2. Alkali Metals and Alkaline Earth Metals:
While highly reactive with water, alkali metals (like sodium (Na) and potassium (K)) and alkaline earth metals (like calcium (Ca) and magnesium (Mg)) also react with strong bases under specific conditions. These reactions are often vigorous and exothermic, requiring careful handling.
3. Transition Metals:
Some transition metals, particularly those with multiple oxidation states, can also react with bases, though their reactivity is generally lower than amphoteric metals. These reactions are often more complex and may require specific conditions, such as elevated temperatures or the presence of oxidizing agents.
Reaction Mechanisms
The reaction mechanisms for metal-base reactions are complex and often involve several steps. A general outline for the reaction of an amphoteric metal with a strong base, such as sodium hydroxide, is as follows:
-
Initial Oxidation: The metal atom loses electrons to form a cation. This is an oxidation process.
-
Hydroxide Ion Attack: Hydroxide ions (OH⁻) from the base attack the metal cation, forming a metal hydroxide.
-
Complex Ion Formation: The metal hydroxide often reacts further with excess hydroxide ions to form complex ions. This complexation process is crucial in stabilizing the reaction products. For example, Al(OH)₃ readily reacts with OH⁻ to form [Al(OH)₄]⁻.
-
Hydrogen Gas Evolution: The reduction process involves the reduction of water molecules to hydrogen gas. This is a characteristic feature of many metal-base reactions.
Factors Influencing Reaction Rate
Several factors influence the rate at which metals react with bases:
-
Concentration of the Base: A higher concentration of the base leads to a faster reaction rate due to a greater number of hydroxide ions available to react with the metal.
-
Temperature: Increasing the temperature generally accelerates the reaction rate, providing more energy for the reaction to proceed.
-
Surface Area of the Metal: A larger surface area (e.g., using powdered metal instead of a solid chunk) increases the contact between the metal and the base, hence speeding up the reaction.
-
Nature of the Metal: The reactivity of the metal itself plays a significant role. More reactive metals react faster.
-
Presence of Impurities: Impurities on the metal's surface can sometimes hinder the reaction rate.
Practical Applications
The reactions of metals with bases have several practical applications:
-
Aluminum Production: The Bayer process, a crucial industrial method for aluminum production, utilizes the reaction of aluminum oxide (Al₂O₃) with sodium hydroxide to dissolve the aluminum and separate it from impurities.
-
Zinc Refining: Similar processes utilize the solubility of zinc in strong bases for refining and purification.
-
Wastewater Treatment: Some metal hydroxides formed in reactions with bases are insoluble and can be used in wastewater treatment to remove heavy metal ions.
-
Chemical Synthesis: Metal-base reactions are employed in the synthesis of various metal compounds, including complex ions and metal hydroxides.
-
Battery Technology: The chemical reactions of some metals with bases are utilized in certain battery technologies.
Safety Precautions
Working with strong bases requires careful consideration of safety measures. Strong bases are corrosive and can cause severe skin and eye burns. Always wear appropriate personal protective equipment (PPE), including gloves, eye protection, and a lab coat. Reactions can be exothermic, so careful control of the reaction temperature is crucial. Proper ventilation is necessary to avoid inhaling any released gases.
Conclusion
The chemical reactions of metals with bases constitute a fascinating and important area of chemistry. While not as ubiquitous as acid-metal reactions, they have crucial industrial applications and demonstrate the complex interplay between redox chemistry, complex ion formation, and the properties of different metals. Understanding these reactions requires a grasp of fundamental chemical concepts and an appreciation for the diverse behaviours of various metals in alkaline environments. Further research into this field continues to unlock new applications and refine existing processes. The continued study of these interactions will undoubtedly lead to further advancements in materials science, industrial processes, and environmental remediation. The information provided in this article serves as a robust introduction to this intricate area, stimulating further exploration and deeper understanding.
Latest Posts
Latest Posts
-
What Is The Difference Between Supportive And Defensive Communication
Mar 24, 2025
-
Is Turnover Number Affected By Substrate Concentration
Mar 24, 2025
-
One Mole Of Any Element Has The Same
Mar 24, 2025
-
Which Of The Following Is A Simple Definition Of Reduction
Mar 24, 2025
-
What Are The Characteristics Of The State
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Chemical Reaction Of Metals With Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
