One Mole Of Any Element Has The Same
Muz Play
Mar 24, 2025 · 6 min read
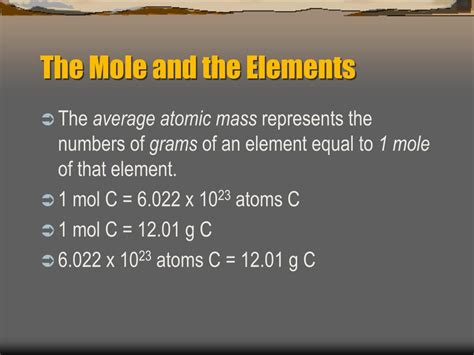
Table of Contents
One Mole of Any Element Has the Same: Exploring the Avogadro Constant and its Implications
The seemingly simple statement, "one mole of any element has the same number of atoms," underpins much of chemistry and our understanding of the material world. This fundamental concept, built around the Avogadro constant, allows us to bridge the gap between the microscopic world of atoms and molecules and the macroscopic world of grams and liters that we experience daily. Let's delve deeper into this crucial concept, exploring its implications and applications in various fields.
Understanding the Mole: A Chemist's Counting Unit
Before we explore the equivalence of one mole of different elements, it's crucial to understand what a mole actually represents. The mole is not a unit of mass or volume, but rather a unit of amount of substance. It's essentially a way for chemists to count incredibly large numbers of atoms, molecules, ions, or other entities. Imagine trying to count the number of grains of sand on a beach – impossible! The mole provides a convenient and standardized way to handle such massive numbers.
The key to the mole lies in the Avogadro constant, approximately 6.022 x 10<sup>23</sup>. This number represents the number of entities (atoms, molecules, etc.) in one mole of any substance. This constant is named after Amedeo Avogadro, an Italian scientist whose work laid the foundation for understanding the relationship between volume and the number of particles in a gas.
Therefore, one mole of carbon atoms contains approximately 6.022 x 10<sup>23</sup> carbon atoms. Similarly, one mole of oxygen atoms contains approximately 6.022 x 10<sup>23</sup> oxygen atoms, and one mole of iron atoms contains the same number of iron atoms. This is the core of the statement: one mole of any element has the same number of atoms.
The Significance of the Avogadro Constant
The Avogadro constant is not just a randomly chosen number; it's a fundamental constant with profound implications:
-
Connecting Atomic Mass to Gram Mass: The periodic table lists the atomic mass of each element. This mass is expressed in atomic mass units (amu), where one amu is approximately the mass of a proton or neutron. The Avogadro constant provides the crucial link between this atomic mass and the gram mass. One mole of an element has a mass in grams numerically equal to its atomic mass. For example, the atomic mass of carbon-12 is 12 amu, so one mole of carbon-12 weighs 12 grams. This allows for easy conversion between the microscopic and macroscopic scales.
-
Stoichiometric Calculations: The mole concept is fundamental to stoichiometry, the study of quantitative relationships between reactants and products in chemical reactions. By using moles, we can accurately predict the amounts of reactants needed and the amounts of products formed in a chemical reaction, ensuring efficient and safe laboratory procedures. Understanding molar ratios enables precise control over chemical processes.
-
Understanding Gas Laws: The Avogadro's Law states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This law, directly related to the Avogadro constant, is crucial for understanding the behavior of gases and is fundamental to many industrial processes that involve gas handling.
-
Applications in Various Fields: The mole concept and the Avogadro constant extend far beyond the confines of a chemistry laboratory. They are crucial in fields like materials science (determining the composition of alloys), environmental science (measuring pollutant concentrations), and biochemistry (analyzing biological molecules).
Beyond Elements: Extending the Mole Concept to Compounds and Molecules
While we've focused on elements, the concept of the mole applies equally to compounds and molecules. One mole of a compound contains 6.022 x 10<sup>23</sup> molecules of that compound. For example, one mole of water (H₂O) contains 6.022 x 10<sup>23</sup> water molecules. The molar mass of a compound is the sum of the atomic masses of all the atoms in its chemical formula.
This allows chemists to perform calculations involving chemical reactions that involve multiple substances. For instance, when balancing a chemical equation, we can use molar ratios to determine the amounts of reactants and products. This precise quantitative approach is essential for industrial chemical processes, ensuring optimal yield and minimizing waste.
Practical Applications: Using Moles in Chemistry
The mole concept is not just a theoretical abstraction; it's a practical tool used daily in chemical laboratories and industries worldwide:
-
Titration: In titration, a solution of known concentration is used to determine the concentration of an unknown solution. Molarity (moles per liter) is the key unit used in these calculations.
-
Gas Analysis: The Ideal Gas Law (PV = nRT) directly utilizes the number of moles (n) to relate pressure (P), volume (V), temperature (T), and the ideal gas constant (R). This law is essential for understanding and controlling gas-phase reactions.
-
Determining Empirical and Molecular Formulas: By analyzing the mass percentages of elements in a compound, chemists can determine its empirical formula (simplest whole-number ratio of atoms). Further analysis, using molar mass, allows for the determination of the molecular formula (actual number of atoms in a molecule).
-
Calculating Reaction Yields: Using stoichiometric calculations with moles, chemists can predict the theoretical yield of a reaction and compare it to the actual yield obtained in the laboratory. This is crucial for optimizing reaction conditions and improving efficiency.
Addressing Misconceptions about the Mole
While the mole is a fundamental concept, some misconceptions are often encountered:
-
The mole is not a measure of mass: While the mass of one mole of a substance is related to its atomic or molecular weight, the mole itself is a measure of the amount of substance, not its mass. Different substances with the same number of moles will have different masses.
-
The Avogadro constant is not arbitrary: While it's a large number, the Avogadro constant is not arbitrary. It is determined experimentally and is fundamental to our understanding of the relationship between the microscopic and macroscopic worlds.
-
The mole is not limited to chemistry: While heavily used in chemistry, the mole concept has applications in various scientific fields, including physics and materials science.
The Future of the Mole and Avogadro's Constant
The Avogadro constant continues to be refined through ongoing research. Improvements in measurement techniques allow for increasingly precise determinations of this fundamental constant, leading to better accuracy in chemical calculations and a deeper understanding of the physical world.
Conclusion: The Importance of the Mole Concept
The simple statement, "one mole of any element has the same number of atoms," is a cornerstone of modern chemistry and beyond. The Avogadro constant and the mole concept provide a crucial bridge between the microscopic world of atoms and molecules and the macroscopic world of experiments and industrial applications. Understanding this concept is fundamental for anyone pursuing studies in chemistry, related fields, or anyone fascinated by the wonders of the material world. The power of the mole lies in its ability to convert between seemingly disparate scales of measurement, enabling precise quantitative analysis that shapes our scientific understanding and drives innovation across many industries. Its importance cannot be overstated, serving as a crucial tool for navigating the complexities of the chemical world.
Latest Posts
Latest Posts
-
Titration Curve Of Hcl And Naoh
Mar 26, 2025
-
List Three Physical Properties Of Water
Mar 26, 2025
-
When A Substance In A Reaction Is Oxidized It
Mar 26, 2025
-
What Happens To Electrons In Metallic Bonding
Mar 26, 2025
-
Label The Types Of Intercellular Junctions
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about One Mole Of Any Element Has The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
