Choose The Correct Names Of The Atoms Or Molecules
Muz Play
Mar 31, 2025 · 8 min read
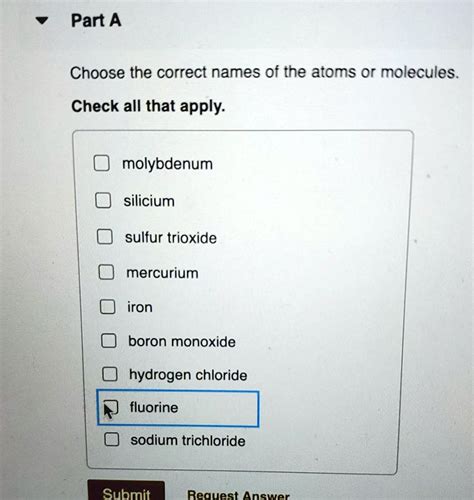
Table of Contents
Choosing the Correct Names of Atoms and Molecules: A Comprehensive Guide
Naming atoms and molecules, also known as chemical nomenclature, is a fundamental aspect of chemistry. A correctly named compound instantly conveys its composition, structure, and properties to other chemists worldwide. This isn't just about memorization; it's about understanding the underlying system that allows for the unambiguous identification of millions of known chemical substances and countless more yet to be discovered. This article will delve into the intricacies of chemical nomenclature, providing a comprehensive guide for accurately naming atoms and molecules.
Understanding the Basics: Atoms vs. Molecules
Before we delve into the complexities of naming, let's establish a clear understanding of the building blocks of matter: atoms and molecules.
Atoms: The Fundamental Building Blocks
Atoms are the smallest units of an element that retain the chemical properties of that element. Each atom is characterized by its atomic number, which represents the number of protons in its nucleus. This number uniquely identifies the element. For example, hydrogen (H) has an atomic number of 1, oxygen (O) has an atomic number of 8, and carbon (C) has an atomic number of 6. The name of the atom is simply the name of the element itself. There's no complex naming system needed here – hydrogen is hydrogen, oxygen is oxygen, and so on.
Molecules: Combinations of Atoms
Molecules are formed when two or more atoms chemically bond together. These bonds can be ionic (involving the transfer of electrons) or covalent (involving the sharing of electrons). The properties of a molecule are often vastly different from the properties of its constituent atoms. For instance, hydrogen (H) is a highly reactive gas, while oxygen (O<sub>2</sub>) is also a gas but supports combustion. Water (H<sub>2</sub>O), a combination of hydrogen and oxygen, is a liquid at room temperature and essential for life. The naming of molecules is significantly more complex than naming atoms and follows specific rules depending on the type of molecule.
Naming Ionic Compounds: A Systematic Approach
Ionic compounds are formed by the electrostatic attraction between oppositely charged ions – cations (positively charged) and anions (negatively charged). The naming conventions for these compounds are relatively straightforward.
Cations: Positively Charged Ions
-
Monatomic cations: These are cations formed from single atoms. Their names are simply the name of the element followed by the word "ion" or, more commonly, just the element name. For example, Na<sup>+</sup> is the sodium ion, K<sup>+</sup> is the potassium ion, and Ca<sup>2+</sup> is the calcium ion. Transition metals, however, can form multiple cations with different charges. In these cases, Roman numerals are used to indicate the charge. For example, Fe<sup>2+</sup> is the iron(II) ion, and Fe<sup>3+</sup> is the iron(III) ion.
-
Polyatomic cations: These are positively charged groups of atoms. Common examples include the ammonium ion (NH<sub>4</sub><sup>+</sup>) and the hydronium ion (H<sub>3</sub>O<sup>+</sup>). These are named according to their specific structures and composition.
Anions: Negatively Charged Ions
-
Monatomic anions: These are formed from single atoms that have gained electrons. Their names are derived from the element name, with the ending changed to "-ide." For example, Cl<sup>-</sup> is the chloride ion, O<sup>2-</sup> is the oxide ion, and S<sup>2-</sup> is the sulfide ion.
-
Polyatomic anions: These are negatively charged groups of atoms. Their names are often less intuitive and must be memorized or readily accessible through a reference. Common examples include sulfate (SO<sub>4</sub><sup>2-</sup>), nitrate (NO<sub>3</sub><sup>-</sup>), phosphate (PO<sub>4</sub><sup>3-</sup>), and carbonate (CO<sub>3</sub><sup>2-</sup>). Prefixes like "bi-" (indicating the addition of a hydrogen ion) and "per-" and "hypo-" (indicating different oxidation states) are used to further differentiate anions.
Naming Ionic Compounds: Putting it Together
To name an ionic compound, the cation is named first, followed by the anion. For example:
- NaCl is sodium chloride.
- MgO is magnesium oxide.
- Al<sub>2</sub>O<sub>3</sub> is aluminum oxide.
- FeCl<sub>3</sub> is iron(III) chloride.
- (NH<sub>4</sub>)<sub>2</sub>SO<sub>4</sub> is ammonium sulfate.
Naming Covalent Compounds: A Matter of Prefixes
Covalent compounds are formed when atoms share electrons to achieve a more stable electron configuration. The naming conventions for covalent compounds differ significantly from those for ionic compounds.
Prefixes Indicate the Number of Atoms
The names of covalent compounds use prefixes to indicate the number of atoms of each element present in the molecule. These prefixes are as follows:
- Mono- (1)
- Di- (2)
- Tri- (3)
- Tetra- (4)
- Penta- (5)
- Hexa- (6)
- Hepta- (7)
- Octa- (8)
- Nona- (9)
- Deca- (10)
The prefix indicating the number of atoms of the first element is followed by the name of that element. The prefix indicating the number of atoms of the second element is followed by the root name of that element with the "-ide" ending.
Examples of Covalent Compound Naming
- CO is carbon monoxide.
- CO<sub>2</sub> is carbon dioxide.
- N<sub>2</sub>O<sub>4</sub> is dinitrogen tetroxide.
- PCl<sub>5</sub> is phosphorus pentachloride.
- SF<sub>6</sub> is sulfur hexafluoride.
Note: The prefix "mono-" is often omitted for the first element if there is only one atom of that element present.
Naming Acids: A Special Case
Acids are compounds that donate protons (H<sup>+</sup> ions) when dissolved in water. They have a specific naming system depending on whether they contain oxygen (oxoacids) or not (binary acids).
Binary Acids: No Oxygen Present
Binary acids are composed of hydrogen and a nonmetal. Their names are formed by using the prefix "hydro-" followed by the root name of the nonmetal with the "-ic" ending, and finally adding the word "acid."
- HCl is hydrochloric acid.
- HBr is hydrobromic acid.
- HI is hydroiodic acid.
Oxoacids: Oxygen Present
Oxoacids contain hydrogen, oxygen, and another nonmetal. Their naming is more complex and depends on the oxidation state of the nonmetal. If the nonmetal has its highest oxidation state, the name ends in "-ic acid." If it has a lower oxidation state, the name ends in "-ous acid."
- HNO<sub>3</sub> is nitric acid (nitrogen in its highest oxidation state).
- HNO<sub>2</sub> is nitrous acid (nitrogen in a lower oxidation state).
- H<sub>2</sub>SO<sub>4</sub> is sulfuric acid (sulfur in its highest oxidation state).
- H<sub>2</sub>SO<sub>3</sub> is sulfurous acid (sulfur in a lower oxidation state).
Organic Compounds: A World of its Own
Organic compounds are carbon-containing compounds (excluding carbonates, carbides, and cyanides). Their nomenclature is far more extensive and complex than inorganic compounds, requiring a separate in-depth study. However, some fundamental concepts are worth mentioning:
-
Alkanes: These are hydrocarbons (containing only carbon and hydrogen) with single bonds. Their names follow a systematic system based on the number of carbon atoms in the chain, using prefixes similar to those used for covalent compounds (meth-, eth-, prop-, but-, pent-, etc.).
-
Alkenes and Alkynes: These hydrocarbons contain double (alkenes) or triple (alkynes) bonds. Their naming involves indicating the position of the double or triple bond within the carbon chain.
-
Functional Groups: Organic molecules often contain specific groups of atoms called functional groups (e.g., alcohols, ketones, carboxylic acids, amines). The presence and location of these functional groups significantly influence the properties and naming of the molecule.
The International Union of Pure and Applied Chemistry (IUPAC) provides comprehensive guidelines for naming organic compounds, which are essential for accurate communication within the field.
Beyond the Basics: Advanced Nomenclature Considerations
The information above covers the fundamentals of chemical nomenclature. However, many nuances and exceptions exist, particularly when dealing with complex molecules and various isomeric forms. Advanced topics include:
-
Isomerism: Molecules with the same molecular formula but different structural arrangements. Nomenclature must distinguish between different isomers (e.g., structural isomers, stereoisomers).
-
Coordination Compounds: Compounds containing complex ions, which require a specific system for naming ligands and central metal atoms.
-
Organometallic Compounds: Compounds containing bonds between carbon atoms and metal atoms, which have their own unique naming rules.
-
Polymers: Large molecules composed of repeating structural units. Nomenclature reflects the repeating unit and its arrangement.
Mastering Chemical Nomenclature: A Continuous Process
Mastering chemical nomenclature is an ongoing process, requiring practice, persistence, and access to reliable resources. The more you encounter and name different compounds, the more comfortable and confident you'll become. Remember, accurate naming is crucial for clear communication in chemistry. By understanding the fundamental principles and systematically applying the appropriate rules, you can confidently choose the correct names for atoms and molecules. Utilizing online resources and chemical handbooks can greatly aid this process. Remember to always double-check your work and refer to established naming conventions to ensure accuracy. The precision of chemical nomenclature is paramount in research, industry, and the overall advancement of chemical science.
Latest Posts
Latest Posts
-
Is The Flu Virus Lytic Or Lysogenic
Apr 02, 2025
-
Is As A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
The Process Of Getting Information Into Memory Is Called
Apr 02, 2025
-
Where Are Nonmetals Located In The Periodic Table
Apr 02, 2025
-
Write An Equation Any Form For The Quadratic Graphed Below
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Choose The Correct Names Of The Atoms Or Molecules . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
