Choose The Kinetic Product Formed During The Reaction Depicted Below.
Muz Play
Mar 30, 2025 · 5 min read
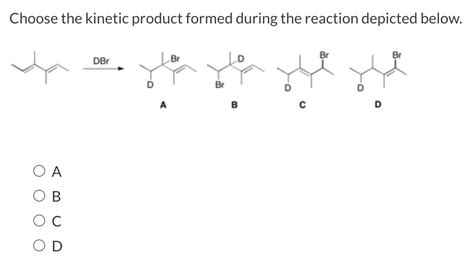
Table of Contents
Choosing the Kinetic Product: A Deep Dive into Reaction Mechanisms and Thermodynamics
The question of which kinetic product is formed during a given reaction is a fundamental concept in organic chemistry. Understanding this requires a thorough grasp of reaction mechanisms, thermodynamics, and the interplay between kinetic and thermodynamic control. This article will explore these concepts in detail, using a hypothetical example reaction to illustrate the principles involved and providing strategies for predicting the major product. We'll examine the factors influencing kinetic versus thermodynamic control and delve into how to identify the kinetic product in various reaction scenarios.
Understanding Kinetic vs. Thermodynamic Control
Before we delve into specific examples, let's clarify the crucial difference between kinetic and thermodynamic control. These terms describe the factors determining the product distribution in a reaction.
-
Kinetic Control: In kinetically controlled reactions, the product distribution is determined by the relative rates of formation of different products. The faster reaction, leading to the faster-forming product, will dominate, even if that product is not the most stable. This often occurs at lower temperatures where the activation energy barrier plays a more significant role. The kinetic product is favored under conditions where the reaction does not have sufficient time to reach equilibrium.
-
Thermodynamic Control: In thermodynamically controlled reactions, the product distribution is determined by the relative stabilities of the products. The most stable product, possessing the lowest Gibbs free energy, will be the major product. This typically occurs at higher temperatures or with longer reaction times, allowing the reaction to reach equilibrium. The system will shift towards the most stable product even if it's formed more slowly.
Factors Affecting Kinetic and Thermodynamic Product Formation
Several factors influence whether a reaction will be kinetically or thermodynamically controlled:
-
Temperature: Higher temperatures generally favor thermodynamic control, providing sufficient energy to overcome activation energy barriers and reach equilibrium. Lower temperatures favor kinetic control, as the reaction may not have enough energy to reach the more stable product.
-
Reaction Time: Longer reaction times allow the reaction to approach equilibrium, favoring the thermodynamic product. Shorter reaction times often lead to the kinetic product predominating.
-
Reversibility of the Reaction: If the reaction is reversible, the thermodynamic product will eventually dominate as the system approaches equilibrium. Irreversible reactions are more likely to be kinetically controlled.
-
Activation Energies: Reactions with large differences in activation energies will show a stronger preference for the kinetically favored product, even at higher temperatures.
-
Solvent Effects: The solvent can significantly influence the reaction rate and stability of intermediates and products, affecting the product distribution.
-
Catalyst: The presence of a catalyst can alter the reaction pathway and the relative rates of formation of different products, influencing the outcome.
Hypothetical Example: Illustrating Kinetic Product Formation
Let's consider a hypothetical addition reaction to an alkene. This type of reaction is commonly studied in relation to kinetic and thermodynamic control, depending on reaction conditions.
Imagine we have an unsymmetrical alkene reacting with a reagent that can add to either side. Two possible products, Product A and Product B, can form. Let's assume:
- Product A: Is formed faster due to a lower activation energy barrier (kinetic product).
- Product B: Is more stable (thermodynamic product). This is typically the result of greater substitution at the position of addition, leading to greater hyperconjugation, and thus lower energy.
Scenario 1: Kinetic Control
Under conditions of low temperature and short reaction time, the reaction will be kinetically controlled. The major product will be Product A, despite its lower stability, due to its faster rate of formation. The lower temperature prevents the reaction from reaching equilibrium and the shorter reaction time doesn't allow sufficient time for Product A to isomerize to Product B.
Scenario 2: Thermodynamic Control
Under conditions of high temperature and long reaction time, the reaction will be thermodynamically controlled. The major product will be Product B, the more stable product, as the system reaches equilibrium. Sufficient energy and time allow the initially formed Product A to undergo isomerization to the more stable Product B.
Identifying the Kinetic Product: Practical Strategies
Several approaches can be used to determine which product is the kinetic product in a given reaction:
-
Analyze Reaction Rates: By measuring the rates of formation of different products under various conditions (temperature, time), you can identify the kinetically favored product as the one formed faster. This often requires sophisticated analytical techniques.
-
Examine Reaction Mechanisms: A detailed understanding of the reaction mechanism helps predict which product will form faster, based on the relative energies of the transition states leading to each product. The product formed through the lower-energy transition state is the kinetic product.
-
Low-Temperature Experiments: Conducting the reaction at low temperatures and short reaction times can help to suppress the formation of the thermodynamic product, making it easier to isolate and identify the kinetic product. Careful control of reaction conditions is necessary.
-
Product Analysis: Using techniques such as NMR, GC-MS, or HPLC to analyze the product mixture and determine the relative amounts of each product can provide evidence for kinetic or thermodynamic control. This is a crucial step to confirm the results of your experiment and hypotheses about reaction mechanisms.
-
Computational Chemistry: Computational methods can be used to calculate the activation energies for the formation of different products, and predict which product will be formed faster. This allows for the prediction of kinetic outcomes, but relies on the accuracy of the computational models used.
Conclusion: Kinetic Control and Reaction Outcomes
Choosing the kinetic product formed during a reaction requires a thorough understanding of reaction mechanisms and thermodynamics. The interplay between these two forces ultimately determines the product distribution. By carefully controlling reaction conditions (temperature, time, solvent) and applying various analytical techniques, one can effectively identify and predict the formation of the kinetic product. Understanding this critical concept is essential for designing and optimizing synthetic routes in organic chemistry. While theoretical calculations can assist in predicting reaction outcomes, experimental verification remains crucial in confirming the identity and relative abundance of the formed products. Remember that context is vital; the same reaction might yield vastly different product distributions under varying conditions, highlighting the dynamic nature of chemical reactions and the importance of reaction conditions in determining which isomer forms as the major product.
Latest Posts
Latest Posts
-
What Is The Z Score For 98 Confidence Interval
Apr 01, 2025
-
Excessive Hormone Production Is Called Hypersecretion
Apr 01, 2025
-
What Are The Simplest Body Structures Considered Alive
Apr 01, 2025
-
Chi Squared Goodness Of Fit Vs Independence
Apr 01, 2025
-
What Is The Symbol For Momentum
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Choose The Kinetic Product Formed During The Reaction Depicted Below. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
