Classify The Characteristics Of Triacylglycerols And Phosphoglycerides
Muz Play
Mar 22, 2025 · 6 min read
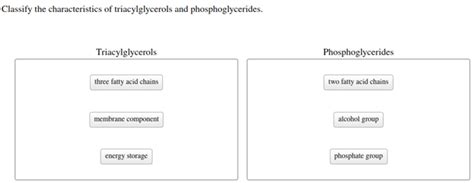
Table of Contents
Classifying the Characteristics of Triacylglycerols and Phosphoglycerides
Lipids, a diverse group of hydrophobic or amphipathic biomolecules, play crucial roles in various biological processes. Among these, triacylglycerols (TAGs) and phosphoglycerides (phospholipids) are particularly important due to their abundance and diverse functions. While both are derived from glycerol, their structural differences lead to vastly different properties and biological roles. This article delves into the detailed classification and characteristics of these two essential lipid classes.
Triacylglycerols: The Energy Storage Champions
Triacylglycerols, also known as triglycerides, are the primary form of energy storage in animals and plants. Their structure is relatively simple yet highly effective for energy storage.
Structural Characteristics of Triacylglycerols
- Glycerol Backbone: A TAG molecule consists of a glycerol backbone, a three-carbon alcohol.
- Esterified Fatty Acids: Three fatty acid molecules are esterified to each of the hydroxyl groups of glycerol. These fatty acids can vary significantly in chain length (typically 12-24 carbons), degree of saturation (saturated, monounsaturated, polyunsaturated), and position of double bonds. This variability is key to the diverse properties of different TAGs.
Classification of Triacylglycerols Based on Fatty Acid Composition
The classification of TAGs is primarily determined by the types of fatty acids they contain. This leads to a huge diversity in their physical and chemical properties.
-
Saturated Triacylglycerols: These contain only saturated fatty acids, meaning they have no double bonds between carbon atoms. They tend to be solid or semi-solid at room temperature (e.g., animal fats like lard and butter). Saturated TAGs are often associated with increased risk of cardiovascular disease when consumed in excess.
-
Unsaturated Triacylglycerols: These contain one or more unsaturated fatty acids, meaning they have one or more double bonds in their fatty acid chains. Unsaturated TAGs can be further classified as monounsaturated (containing one double bond) or polyunsaturated (containing multiple double bonds). They tend to be liquid at room temperature (e.g., vegetable oils like olive oil and sunflower oil). Unsaturated fatty acids, particularly polyunsaturated ones like omega-3 and omega-6 fatty acids, are essential for human health.
-
Mixed Triacylglycerols: Most naturally occurring TAGs are mixed triacylglycerols, containing a combination of saturated and unsaturated fatty acids. The ratio of saturated to unsaturated fatty acids influences the melting point and physical state of the TAG.
Physical and Chemical Properties of Triacylglycerols
-
Hydrophobicity: TAGs are highly hydrophobic, meaning they are insoluble in water. This characteristic is crucial for their role in energy storage, as they don't interfere with the aqueous environment of cells.
-
Melting Point: The melting point of a TAG is influenced by the length and degree of saturation of its fatty acids. Saturated TAGs generally have higher melting points than unsaturated TAGs.
-
Hydrolysis: TAGs can be hydrolyzed by lipases, enzymes that break the ester bonds between glycerol and fatty acids, releasing free fatty acids and glycerol. This process is crucial for energy mobilization in the body.
-
Saponification: TAGs can undergo saponification, a reaction with strong bases (like NaOH or KOH) to produce glycerol and fatty acid salts (soaps). This reaction is the basis of soap making.
Phosphoglycerides: The Structural Components of Membranes
Phosphoglycerides, also known as phospholipids, are the major structural components of cell membranes. Unlike TAGs, they possess amphipathic properties, meaning they have both hydrophilic (water-loving) and hydrophobic (water-fearing) regions.
Structural Characteristics of Phosphoglycerides
-
Glycerol Backbone: Similar to TAGs, phosphoglycerides also possess a glycerol backbone.
-
Esterified Fatty Acids: Two fatty acids are esterified to the first and second hydroxyl groups of glycerol. These fatty acids, like in TAGs, can vary in chain length and saturation. The fatty acid at the sn-1 position is usually saturated, while the fatty acid at the sn-2 position is usually unsaturated.
-
Phosphate Group: A phosphate group is esterified to the third hydroxyl group of glycerol. This phosphate group is highly polar and hydrophilic.
-
Polar Head Group: A polar head group is attached to the phosphate group. This head group can vary widely, defining different types of phosphoglycerides. Common head groups include choline (phosphatidylcholine), ethanolamine (phosphatidylethanolamine), serine (phosphatidylserine), and inositol (phosphatidylinositol). The polar head group is hydrophilic, contributing to the amphipathic nature of phosphoglycerides.
Classification of Phosphoglycerides Based on Polar Head Groups
The classification of phosphoglycerides primarily depends on their polar head groups.
-
Phosphatidylcholine (PC): Contains choline as the polar head group. It's abundant in cell membranes and is a major component of lung surfactant.
-
Phosphatidylethanolamine (PE): Contains ethanolamine as the polar head group. It's also common in cell membranes and plays a role in membrane fusion and fission.
-
Phosphatidylserine (PS): Contains serine as the polar head group. It's usually located in the inner leaflet of the plasma membrane and plays a role in cell signaling and apoptosis.
-
Phosphatidylinositol (PI): Contains inositol as the polar head group. It is a precursor to various signaling molecules involved in cell signaling pathways.
-
Phosphatidylglycerol (PG): Contains glycerol as the polar head group. It is abundant in bacterial membranes and the inner mitochondrial membrane.
-
Cardiolipin: A unique phosphoglyceride with four fatty acids and two phosphate groups. It's predominantly found in the inner mitochondrial membrane and is important for mitochondrial function.
Physical and Chemical Properties of Phosphoglycerides
-
Amphipathic Nature: The most striking characteristic of phosphoglycerides is their amphipathic nature. The two fatty acid tails are hydrophobic, while the polar head group is hydrophilic. This allows them to form bilayers in aqueous environments, the fundamental structure of cell membranes.
-
Formation of Bilayers and Micelles: In aqueous solutions, phosphoglycerides spontaneously form bilayers or micelles. Bilayers are stable structures where the hydrophobic tails are oriented towards the interior, away from water, while the hydrophilic heads are oriented towards the aqueous environment. Micelles are spherical structures formed at lower concentrations.
-
Membrane Fluidity: The fluidity of cell membranes is influenced by the fatty acid composition of phosphoglycerides. The presence of unsaturated fatty acids increases membrane fluidity, while saturated fatty acids decrease it.
-
Membrane Permeability: Phospholipid bilayers are selectively permeable, meaning they control the passage of molecules across the membrane. Small, nonpolar molecules can pass through more easily than large or polar molecules.
Comparing Triacylglycerols and Phosphoglycerides: A Summary Table
| Feature | Triacylglycerols | Phosphoglycerides |
|---|---|---|
| Main Function | Energy storage | Membrane structure |
| Glycerol Esters | Three fatty acids | Two fatty acids, one phosphate, one polar head group |
| Polarity | Nonpolar, hydrophobic | Amphipathic (hydrophobic and hydrophilic) |
| Water Solubility | Insoluble | Insoluble (forms bilayers or micelles in water) |
| Melting Point | Varies with fatty acid composition | Varies with fatty acid and head group composition |
| Biological Role | Energy source, insulation | Cell membrane structure, signaling |
| Location | Adipose tissue, seeds | Cell membranes, organelles |
Conclusion
Triacylglycerols and phosphoglycerides, despite sharing a common glycerol backbone, exhibit distinct characteristics that reflect their differing biological roles. TAGs serve primarily as energy storage molecules, characterized by their hydrophobicity and varied fatty acid composition. Phosphoglycerides, on the other hand, are essential structural components of cell membranes, distinguished by their amphipathic nature and diverse polar head groups. Understanding the structural and functional properties of these two lipid classes is fundamental to grasping the complexities of cellular biology and metabolism. Further research continues to uncover the nuanced roles of specific fatty acid compositions and head groups in health and disease, highlighting their importance in maintaining cellular integrity and function. The continuing exploration into the specific interactions of different lipid species and their contributions to overall cellular physiology holds significant promise for future advancements in medicine and biotechnology.
Latest Posts
Latest Posts
-
Determine Whether The Graph Is The Graph Of A Function
Mar 22, 2025
-
Lewis Structure Practice Worksheet And Answers
Mar 22, 2025
-
The Cell The Basic Unit Of Life
Mar 22, 2025
-
Power Series Solution Of Ordinary Differential Equations
Mar 22, 2025
-
How Many Electrons Does Li2 Have
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Classify The Characteristics Of Triacylglycerols And Phosphoglycerides . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
