Co Is What Type Of Bond
Muz Play
Mar 31, 2025 · 5 min read
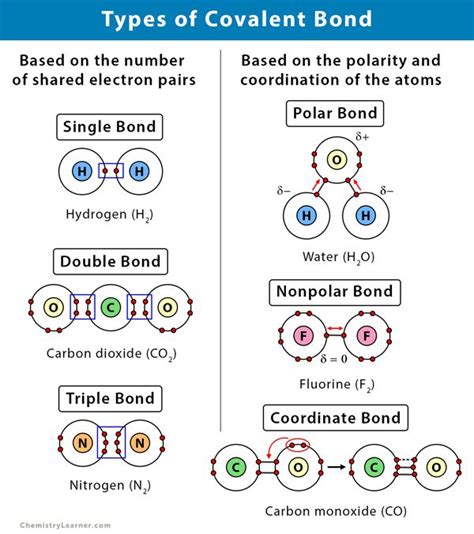
Table of Contents
CO: Exploring the Nature of the Carbon-Oxygen Bond
The carbon-oxygen bond (C=O) found in carbon monoxide (CO) is a fascinating example of chemical bonding, showcasing a unique interplay of covalent and coordinate bonding characteristics. Understanding its nature requires delving into the concepts of molecular orbital theory, bond order, and the influence of electronegativity. This exploration will dissect the intricacies of the CO bond, comparing it to other carbon-oxygen bonds and examining its implications in various fields, from chemistry and biology to industrial applications.
Delving into the Covalent Bond
At its core, the bond in carbon monoxide is primarily a covalent bond. This means that carbon and oxygen atoms share electrons to achieve a more stable electron configuration. Each atom contributes electrons to the shared pool, forming molecular orbitals that encompass both atoms. However, the simple picture of a purely covalent bond doesn't fully capture the reality of the CO bond's unique properties. The significant difference in electronegativity between carbon and oxygen introduces complexity.
Electronegativity and Bond Polarity
Oxygen (O) is significantly more electronegative than carbon (C). Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond. This difference in electronegativity leads to a polar covalent bond, where the electron density is unevenly distributed. The oxygen atom attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on the oxygen and a partial positive charge (δ+) on the carbon. This polarity significantly influences the molecule's reactivity and interactions with other molecules.
The Role of Coordinate Bonding (Dative Covalent Bond)
To achieve a stable octet, each atom needs eight electrons in its valence shell. While the covalent bond contributes significantly, a deeper understanding requires considering the coordinate bond or dative covalent bond. This type of bond involves one atom donating both electrons to the shared pair. In CO, the carbon atom essentially donates a lone pair of electrons to the oxygen atom, creating a coordinate bond alongside the covalent bond.
Molecular Orbital Theory and Bond Order
Molecular orbital theory (MOT) provides a more sophisticated description of the bonding in CO. It describes the formation of molecular orbitals through the combination of atomic orbitals. In CO, the combination of atomic orbitals leads to the formation of sigma (σ) and pi (π) bonding molecular orbitals. The presence of both sigma and pi bonds contributes to the high bond order of CO.
Bond order is a measure of the number of chemical bonds between a pair of atoms. It's calculated as half the difference between the number of electrons in bonding and antibonding molecular orbitals. For CO, the bond order is 3, indicating a very strong triple bond consisting of one sigma (σ) bond and two pi (π) bonds. This high bond order explains the exceptional strength and shortness of the C≡O bond.
Comparing CO to other Carbon-Oxygen Bonds
The C≡O triple bond in carbon monoxide is significantly different from other carbon-oxygen bonds, such as those found in carbon dioxide (CO₂) and carbonyl compounds. While CO₂ also involves carbon-oxygen bonds, they are double bonds (C=O) and the molecule is linear, resulting in different properties. In carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids), the C=O bond is part of a larger functional group and its reactivity is influenced by the neighboring atoms and functional groups.
The difference in bond order directly impacts the bond length and bond energy. The triple bond in CO is shorter and stronger than the double bond in CO₂ or carbonyl compounds. This strength contributes to the relative inertness of carbon monoxide in certain reactions. The high bond energy means that a significant amount of energy is required to break the bond, making CO relatively stable under various conditions.
Biological and Industrial Significance
The unique properties of the CO bond have significant implications across various fields:
Biological Aspects:
-
Toxicity: Despite its stability, CO's strong affinity for hemoglobin makes it highly toxic. Hemoglobin's primary function is to transport oxygen in the blood. CO binds to hemoglobin much more strongly than oxygen, preventing oxygen transport and causing hypoxia (oxygen deprivation).
-
Signaling Molecule: Surprisingly, at low concentrations, CO is emerging as an important signaling molecule in biological systems. It plays a role in regulating various physiological processes, including vasodilation (widening of blood vessels) and inflammation. The precise mechanisms of CO's signaling actions are still under investigation, but research suggests its interaction with specific enzymes and proteins.
Industrial Applications:
-
Fuel Source: Carbon monoxide is a crucial intermediate in many industrial processes. It's used as a reducing agent in metallurgy, helping to extract metals from their ores.
-
Chemical Synthesis: It serves as a building block in the synthesis of numerous organic compounds, demonstrating its versatility in chemical reactions. The reactivity of the C≡O triple bond makes it a valuable synthon (a starting material in chemical synthesis).
-
Industrial Gases: Carbon monoxide is a component of several industrial gas mixtures, used in various applications. The controlled use of CO's reactive properties in industrial processes is essential for its safe and efficient application.
Conclusion
The carbon-oxygen bond in carbon monoxide is a compelling example of chemical bonding, showcasing the intricate interplay of covalent and coordinate bonding. The high bond order, stemming from a combination of sigma and pi bonds, leads to its exceptional strength and relative inertness. However, its high affinity for hemoglobin makes it highly toxic at higher concentrations. Despite its toxicity, CO is also recognized as an important signaling molecule in biological systems and plays a crucial role in various industrial processes. Understanding the nature of this unique bond is essential for both comprehending its biological roles and harnessing its industrial applications. The study of CO continues to reveal new facets of its chemical behaviour, highlighting the rich and complex landscape of chemical bonding. Future research will undoubtedly continue to uncover new insights into the significance of this intriguing molecule.
Latest Posts
Latest Posts
-
Choose The Most Likely Correlation Value For This Scatterplot
Apr 01, 2025
-
Which Is The Noble Gas Notation For Chlorine
Apr 01, 2025
-
Describe The Role Of Carbon In Biological Systems
Apr 01, 2025
-
How Is The Domain Rank Different From Other Ranks
Apr 01, 2025
-
Wilcoxon Signed Rank Test In Sas
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Co Is What Type Of Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
