Complete The Overall Reaction Catalyzed By The Pyruvate Dehydrogenase Complex
Muz Play
Mar 30, 2025 · 5 min read
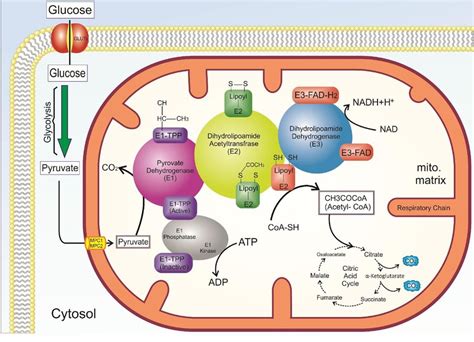
Table of Contents
The Pyruvate Dehydrogenase Complex: A Complete Overview of the Reaction
The pyruvate dehydrogenase complex (PDC) stands as a pivotal enzyme complex in cellular metabolism, acting as a crucial bridge between glycolysis and the citric acid cycle (Krebs cycle). Its primary function is the oxidative decarboxylation of pyruvate, a three-carbon molecule derived from glucose metabolism, into acetyl-CoA, a two-carbon molecule that enters the citric acid cycle for further oxidation and ATP generation. Understanding the complete reaction catalyzed by this remarkable complex is essential for grasping fundamental metabolic processes. This comprehensive article will delve into the intricate details of the PDC reaction, exploring its individual steps, regulatory mechanisms, and clinical significance.
The Overall Reaction: A Summary
Before diving into the mechanistic intricacies, let's summarize the overall reaction catalyzed by the pyruvate dehydrogenase complex:
Pyruvate + CoA-SH + NAD⁺ → Acetyl-CoA + CO₂ + NADH + H⁺
This seemingly simple equation belies the complex series of reactions facilitated by the PDC. The reaction involves the irreversible conversion of pyruvate into acetyl-CoA, a crucial step that commits pyruvate to complete oxidation within the mitochondria. Let's break down the individual components:
- Pyruvate: The substrate, originating from glycolysis.
- CoA-SH (Coenzyme A): A critical cofactor that carries the acetyl group.
- NAD⁺ (Nicotinamide adenine dinucleotide): An electron acceptor, reduced to NADH in the process.
- Acetyl-CoA: The product, entering the citric acid cycle.
- CO₂ (Carbon dioxide): A byproduct released during decarboxylation.
- NADH + H⁺: Reduced NAD⁺, carrying high-energy electrons to the electron transport chain.
The Multi-Enzyme Nature of the PDC
The PDC is not a single enzyme but rather a large, multi-enzyme complex composed of three distinct enzymes:
- Pyruvate dehydrogenase (E1): Catalyzes the decarboxylation of pyruvate.
- Dihydrolipoyl transacetylase (E2): Transfers the acetyl group to CoA-SH.
- Dihydrolipoyl dehydrogenase (E3): Regenerates the oxidized form of the lipoyl group and reduces NAD⁺ to NADH.
These three enzymes, along with five coenzymes, work in a highly coordinated manner to achieve the overall reaction.
Step-by-Step Mechanism: Unraveling the Catalytic Process
The reaction catalyzed by the PDC unfolds in five distinct steps:
Step 1: Decarboxylation of Pyruvate by E1
This initial step is catalyzed by pyruvate dehydrogenase (E1), a thiamine pyrophosphate (TPP)-dependent enzyme. TPP acts as a crucial cofactor, forming a carbanion intermediate that attacks the carbonyl group of pyruvate. This leads to the decarboxylation of pyruvate, releasing CO₂ and forming a hydroxyethyl-TPP intermediate bound to E1.
Step 2: Transfer of the Hydroxyethyl Group to Lipoamide by E2
The hydroxyethyl group is then transferred from TPP to the lipoamide prosthetic group of dihydrolipoyl transacetylase (E2). Lipoamide is a flexible molecule containing a disulfide bond that accepts the hydroxyethyl group, forming an acetyl-lipoamide intermediate. This step involves a critical oxidation of the hydroxyethyl group to an acetyl group.
Step 3: Transacetylation to CoA-SH by E2
The acetyl group is then transferred from the lipoamide to coenzyme A (CoA-SH), generating acetyl-CoA. This transacetylation reaction is also catalyzed by E2. The resulting lipoic acid is now in its reduced dihydrolipoyl form.
Step 4: Regeneration of Oxidized Lipoamide by E3
Dihydrolipoyl dehydrogenase (E3) is responsible for regenerating the oxidized form of lipoamide. This crucial step involves the transfer of electrons from the reduced dihydrolipoyl group to FAD (flavin adenine dinucleotide), a coenzyme of E3. This reduces FAD to FADH₂.
Step 5: Reduction of NAD⁺ by E3
Finally, the electrons from FADH₂ are transferred to NAD⁺, reducing it to NADH. This step regenerates the oxidized form of FAD, completing the catalytic cycle.
Coenzymes: The Essential Players
Five essential coenzymes are involved in the PDC reaction, each playing a distinct role:
- Thiamine pyrophosphate (TPP): Crucial for decarboxylation of pyruvate by E1.
- Lipoic acid: Acts as an acyl group carrier between E1 and E2.
- Coenzyme A (CoA-SH): Accepts the acetyl group to form acetyl-CoA.
- FAD (Flavin adenine dinucleotide): Electron carrier in E3.
- NAD⁺ (Nicotinamide adenine dinucleotide): Final electron acceptor, forming NADH.
Regulation of the Pyruvate Dehydrogenase Complex
The activity of the PDC is tightly regulated to meet the cell's energy demands. Several mechanisms control its activity:
-
Product Inhibition: Acetyl-CoA and NADH, the products of the reaction, inhibit the PDC. High levels of these molecules signal sufficient energy stores, reducing the need for further pyruvate oxidation.
-
Substrate Availability: The availability of pyruvate, CoA-SH, and NAD⁺ also regulates the PDC's activity. Low levels of these substrates limit the reaction rate.
-
Phosphorylation-Dephosphorylation: Pyruvate dehydrogenase kinase (PDK) phosphorylates E1, leading to its inactivation. Pyruvate dehydrogenase phosphatase (PDP) dephosphorylates E1, reactivating it. The balance between these two enzymes is influenced by energy levels and various metabolic signals, including ATP, ADP, pyruvate, acetyl-CoA, and NADH. High energy charge favors phosphorylation and inactivation, while low energy charge favors dephosphorylation and activation.
Clinical Significance: Linking PDC Dysfunction to Disease
Dysfunction of the PDC can lead to several metabolic disorders, collectively known as pyruvate dehydrogenase complex deficiency (PDCD). This deficiency can result from mutations in the genes encoding the PDC enzymes or their associated coenzymes. The severity of PDCD varies greatly, with symptoms ranging from mild lactic acidosis to severe neurological impairments. Diagnosing PDCD often involves measuring pyruvate and lactate levels in blood and other body fluids. Treatment focuses on managing symptoms and providing alternative energy sources.
Conclusion: A Vital Metabolic Hub
The pyruvate dehydrogenase complex is a remarkable example of metabolic coordination, catalyzing a critical step in cellular energy production. Its multi-enzyme nature, intricate mechanisms, and sophisticated regulation highlight the complexity and elegance of cellular metabolism. Understanding the complete reaction catalyzed by the PDC is essential for appreciating the intricate interplay of metabolic pathways and the implications of its dysfunction in various clinical contexts. Further research continues to unravel the detailed mechanisms of PDC regulation and its role in various physiological and pathological conditions, promising advances in diagnosis and treatment of related disorders. The ongoing exploration of this vital metabolic hub promises to reveal even more insights into the fundamental processes underpinning life itself.
Latest Posts
Latest Posts
-
Hair Like Outgrowths That Attach To Bacteria
Apr 01, 2025
-
Which Of The Following Is An Example Of Ottonian Architecture
Apr 01, 2025
-
Antimicrobial Sensitivity Testing The Kirby Bauer Method
Apr 01, 2025
-
Calculate Ph Of A Weak Base
Apr 01, 2025
-
Example Of A Summary And Response Essay
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Complete The Overall Reaction Catalyzed By The Pyruvate Dehydrogenase Complex . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
