Conjugate Base Of A Weak Acid
Muz Play
Mar 16, 2025 · 6 min read
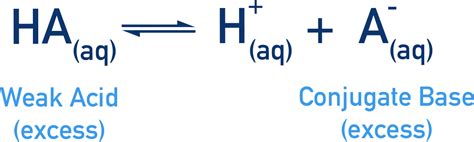
Table of Contents
Conjugate Bases of Weak Acids: A Deep Dive
Understanding conjugate bases is crucial for mastering acid-base chemistry. This article delves into the properties, behavior, and significance of conjugate bases derived from weak acids, providing a comprehensive overview for students and enthusiasts alike. We'll explore their relationship to weak acids, their impact on pH, and their applications in various fields.
What is a Conjugate Base?
An acid, by the Brønsted-Lowry definition, is a proton (H⁺) donor. When an acid donates a proton, it forms its conjugate base. The conjugate base is essentially the acid minus a proton. The relationship is reciprocal: the conjugate base can accept a proton to reform the original acid. This acid-base pair is connected by a single proton transfer.
Example: Consider acetic acid (CH₃COOH), a common weak acid. When it donates a proton, it forms its conjugate base, the acetate ion (CH₃COO⁻).
CH₃COOH ⇌ CH₃COO⁻ + H⁺
In this equation, CH₃COOH is the acid, CH₃COO⁻ is its conjugate base, and H⁺ is the proton. The double arrow (⇌) indicates that the reaction is an equilibrium, meaning both the forward and reverse reactions occur simultaneously.
Weak Acids and Their Conjugate Bases: A Special Relationship
The strength of a weak acid directly influences the properties of its conjugate base. Weak acids, unlike strong acids, only partially dissociate in water. This means that a significant portion of the weak acid remains undissociated in solution. This partial dissociation leads to the formation of a conjugate base that exhibits specific characteristics:
1. Basic Nature:
The conjugate base of a weak acid is itself a weak base. This is because it can accept a proton from water molecules to regenerate the weak acid and produce hydroxide ions (OH⁻). This process leads to an increase in the solution's pH, making the solution slightly basic.
CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻
The extent to which this reaction proceeds is determined by the strength of the weak acid. A weaker acid will have a stronger conjugate base, meaning it will react more readily with water to produce hydroxide ions.
2. Hydrolysis:
The reaction of a conjugate base with water to produce hydroxide ions is termed hydrolysis. The extent of hydrolysis determines the basicity of the conjugate base. A higher degree of hydrolysis indicates a stronger conjugate base. The equilibrium constant for this hydrolysis reaction is called the base hydrolysis constant (K<sub>b</sub>).
3. Relationship between K<sub>a</sub> and K<sub>b</sub>:
The acid dissociation constant (K<sub>a</sub>) and the base hydrolysis constant (K<sub>b</sub>) are related through the ion product constant of water (K<sub>w</sub>):
K<sub>a</sub> * K<sub>b</sub> = K<sub>w</sub> = 1.0 x 10⁻¹⁴ at 25°C
This equation highlights the inverse relationship between the strength of a weak acid and its conjugate base. A weaker acid (smaller K<sub>a</sub>) will have a stronger conjugate base (larger K<sub>b</sub>), and vice versa.
Factors Influencing the Strength of a Conjugate Base
Several factors influence the strength of a conjugate base:
1. Electronegativity:
The electronegativity of the atoms in the conjugate base affects its ability to hold onto the negative charge. A more electronegative atom will more effectively stabilize the negative charge, making the conjugate base weaker.
2. Size and Resonance:
Larger conjugate bases and those with resonance structures can better delocalize the negative charge, making them weaker bases. This is because the negative charge is spread over a larger area, reducing its concentration and reactivity.
3. Inductive Effects:
Electron-withdrawing groups can stabilize the negative charge on the conjugate base, making it a weaker base. Conversely, electron-donating groups destabilize the negative charge, resulting in a stronger conjugate base.
Calculating pH of Solutions Containing Conjugate Bases
The pH of a solution containing a conjugate base can be calculated using the following approaches:
1. Using the K<sub>b</sub> value:
If the K<sub>b</sub> value is known, the hydroxide ion concentration [OH⁻] can be calculated using an ICE (Initial, Change, Equilibrium) table and the equilibrium expression for the hydrolysis reaction. The pOH can then be calculated using:
pOH = -log[OH⁻]
Finally, the pH can be determined using:
pH + pOH = 14
2. Using the K<sub>a</sub> value and the relationship between K<sub>a</sub> and K<sub>b</sub>:
If the K<sub>a</sub> of the parent weak acid is known, K<sub>b</sub> can be calculated using the equation:
K<sub>b</sub> = K<sub>w</sub> / K<sub>a</sub>
Then, proceed with the calculation as described above.
Applications of Conjugate Bases
Conjugate bases of weak acids find widespread applications in various fields:
1. Buffer Solutions:
A crucial application involves creating buffer solutions. Buffer solutions resist changes in pH upon the addition of small amounts of acid or base. They are typically composed of a weak acid and its conjugate base (or a weak base and its conjugate acid). The conjugate base neutralizes added acid, while the weak acid neutralizes added base, maintaining a relatively constant pH. This is essential in many biological systems and chemical processes.
2. Pharmaceutical Industry:
Many pharmaceuticals utilize conjugate bases in their formulations. The properties of conjugate bases, such as their solubility and ability to act as weak bases, can significantly impact drug efficacy and delivery.
3. Environmental Science:
Conjugate bases play a role in understanding and managing environmental processes such as water chemistry and soil acidity. The presence and concentration of conjugate bases influence the overall pH and chemical equilibrium in various environmental systems.
Common Examples of Weak Acids and Their Conjugate Bases
| Weak Acid | Formula | Conjugate Base | Formula |
|---|---|---|---|
| Acetic acid | CH₃COOH | Acetate ion | CH₃COO⁻ |
| Formic acid | HCOOH | Formate ion | HCOO⁻ |
| Benzoic acid | C₆H₅COOH | Benzoate ion | C₆H₅COO⁻ |
| Ammonium ion | NH₄⁺ | Ammonia | NH₃ |
| Carbonic acid | H₂CO₃ | Bicarbonate ion | HCO₃⁻ |
| Phosphoric acid | H₃PO₄ | Dihydrogen phosphate ion | H₂PO₄⁻ |
| Hydrogen phosphate ion | H₂PO₄⁻ | Monohydrogen phosphate ion | HPO₄²⁻ |
| Monohydrogen phosphate ion | HPO₄²⁻ | Phosphate ion | PO₄³⁻ |
This table provides a glimpse into the diversity of weak acids and their corresponding conjugate bases, highlighting their prevalence in various chemical contexts.
Conclusion
The concept of conjugate bases of weak acids is fundamental to understanding acid-base chemistry. Their properties, including their basic nature, hydrolysis, and relationship to their parent acids, are crucial for predicting and manipulating the pH of solutions. From buffer solutions to pharmaceutical formulations and environmental science, conjugate bases play essential roles in various scientific and technological applications. A thorough understanding of their behavior and characteristics is indispensable for anyone working in these fields. This detailed explanation provides a solid foundation for further exploration into the intricacies of acid-base equilibria and their implications. Remember to practice calculations and familiarize yourself with different examples to solidify your understanding of this crucial concept.
Latest Posts
Latest Posts
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
-
Why Do Ions Travel Back And Forth In Orbitrap
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Conjugate Base Of A Weak Acid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
