Cual Es El Punto De Congelacion En Grados Fahrenheit
Muz Play
Mar 16, 2025 · 5 min read
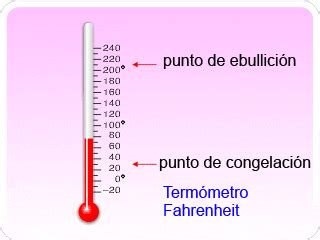
Table of Contents
What is the Freezing Point in Fahrenheit? Understanding Temperature and Phase Transitions
The freezing point of water, a fundamental concept in science and everyday life, is a crucial reference point for understanding temperature scales. While many parts of the world utilize the Celsius scale, the Fahrenheit scale remains prevalent, especially in the United States. This article will delve deep into the freezing point of water in Fahrenheit, exploring its significance, the science behind it, and its practical applications.
Defining the Freezing Point
The freezing point is the temperature at which a liquid substance transitions to a solid state. This transition, known as freezing or solidification, occurs when the kinetic energy of the liquid molecules decreases sufficiently, allowing them to form a stable, ordered structure characteristic of a solid. For pure water at standard atmospheric pressure, this transition happens at a precise temperature.
The Freezing Point of Water in Fahrenheit: 32°F
This is a critical value to remember. 32°F is the temperature at which liquid water begins to freeze into ice under standard atmospheric pressure (1 atmosphere or 101.325 kPa). Any temperature below 32°F will cause further freezing of the water, while temperatures above 32°F will maintain water in its liquid state (assuming no other factors are involved like impurities or pressure changes).
Understanding Temperature Scales
To fully grasp the significance of 32°F, it's essential to understand the different temperature scales and their interconversions.
-
Fahrenheit (°F): Developed by Daniel Gabriel Fahrenheit in the early 18th century, this scale uses 32°F as the freezing point of water and 212°F as its boiling point. The scale is based on the freezing point of a brine solution (a mixture of water and salt) and the human body temperature.
-
Celsius (°C): Also known as the centigrade scale, Celsius uses 0°C for the freezing point of water and 100°C for its boiling point. This scale is more widely used in scientific contexts and internationally.
-
Kelvin (K): This is the absolute temperature scale, where 0 K represents absolute zero – the theoretical point where all molecular motion ceases. The Kelvin scale is widely used in scientific applications, particularly in thermodynamics.
Converting Between Scales
Converting between Fahrenheit and Celsius is crucial for understanding temperature values across different systems. The formulas are:
- °C to °F: (°C × 9/5) + 32 = °F
- °F to °C: (°F - 32) × 5/9 = °C
Therefore, 32°F is equivalent to 0°C.
The Science Behind Freezing: Molecular Behavior
The freezing of water is a fascinating example of phase transition driven by intermolecular forces. Water molecules are polar, meaning they have a slightly positive and slightly negative end. These polar molecules attract each other through hydrogen bonds, weak electrostatic interactions.
At temperatures above 32°F, water molecules possess sufficient kinetic energy to overcome these hydrogen bonds, resulting in a relatively free-flowing liquid state. As the temperature drops towards 32°F, the kinetic energy decreases. The hydrogen bonds begin to dominate, forcing the water molecules into a more ordered, crystalline structure – ice.
Factors Affecting the Freezing Point
While 32°F is the freezing point of pure water under standard conditions, several factors can influence the actual freezing temperature:
-
Pressure: Increasing pressure slightly lowers the freezing point of water. This is a unique property of water, unlike most substances where increased pressure raises the freezing point.
-
Impurities: Dissolved substances (like salt) in water lower its freezing point. This is why salt is used to de-ice roads in winter. The presence of impurities disrupts the formation of the ice crystal lattice, requiring a lower temperature for freezing.
-
Supercooling: Under certain conditions, water can remain liquid even below 32°F. This is known as supercooling and requires the absence of nucleation sites (surfaces where ice crystals can begin to form). A slight disturbance or the introduction of a nucleation site will trigger rapid freezing.
Practical Applications of the Freezing Point
The freezing point of water at 32°F has numerous practical applications across various fields:
-
Food Preservation: Freezing food at or below 32°F is a common method for preservation, slowing down microbial growth and enzymatic activity.
-
Construction: Understanding the freezing point is crucial in construction, particularly in cold climates, to prevent water damage from freezing and thawing cycles.
-
Weather Forecasting: The freezing point is a fundamental element in weather forecasting, determining the likelihood of snow, ice, and freezing rain.
-
Medicine: Freezing is used in cryosurgery, a medical procedure that employs extremely low temperatures to destroy abnormal tissues.
The Freezing Point and Everyday Life
The freezing point of water at 32°F directly impacts our daily lives, from simple tasks like making ice cubes to more complex considerations like winter driving safety and agriculture. Understanding this temperature is essential for effective planning and risk mitigation.
-
Winter Driving: Knowing the freezing point allows for safer winter driving practices, including appropriate tire preparation and awareness of icy road conditions.
-
Gardening: Farmers and gardeners must consider the freezing point to protect plants from frost damage, using methods like covering plants or employing frost protection techniques.
-
Food Safety: Proper food storage and handling require awareness of the freezing point to ensure food safety and prevent spoilage.
Beyond Water: Freezing Points of Other Substances
While this article has focused on the freezing point of water, it's important to remember that every substance has its own unique freezing point. These freezing points vary greatly depending on the substance's molecular structure and intermolecular forces.
For example, the freezing point of ethanol (alcohol) is significantly lower than that of water, while the freezing point of mercury is much lower still. These differences are crucial in various applications, from industrial processes to the design of specialized coolants.
Conclusion: The Significance of 32°F
The freezing point of water at 32°F is a fundamental concept with far-reaching implications. Understanding this value and the underlying science behind it is crucial for a wide range of applications, from everyday tasks to advanced scientific research. This understanding enables us to improve our lives, protect our environment, and make informed decisions in various aspects of our lives. The significance of 32°F extends far beyond a simple numerical value; it represents a critical point in the transition of matter and a foundational element in our comprehension of the world around us. By understanding this crucial temperature, we can better navigate the complexities of our environment and harness the power of phase transitions for numerous practical applications.
Latest Posts
Latest Posts
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Cual Es El Punto De Congelacion En Grados Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
