Delta G Delta H Delta S Table
Muz Play
Mar 23, 2025 · 5 min read
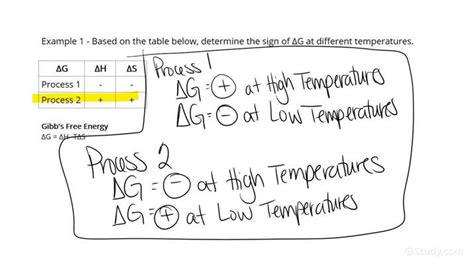
Table of Contents
Understanding Gibbs Free Energy, Enthalpy, and Entropy: A Comprehensive Guide with Table Examples
Thermodynamics, a cornerstone of chemistry and physics, helps us understand energy transformations in systems. Three crucial state functions – Gibbs Free Energy (ΔG), Enthalpy (ΔH), and Entropy (ΔS) – are vital in predicting the spontaneity and equilibrium of chemical and physical processes. This article delves deep into the significance of each, their interrelationship, and provides illustrative examples using a tabular format for clarity.
What is Gibbs Free Energy (ΔG)?
Gibbs Free Energy, denoted by ΔG, measures the maximum reversible work that may be performed by a thermodynamic system at a constant temperature and pressure. It's a crucial predictor of a reaction's spontaneity. A negative ΔG indicates a spontaneous process (occurs naturally without external intervention), while a positive ΔG signifies a non-spontaneous process (requires external energy input). A ΔG of zero implies the system is at equilibrium.
Key characteristics of ΔG:
- Spontaneity: The primary indicator of a reaction's direction.
- Temperature-dependent: ΔG's value can change with temperature alterations.
- Pressure-dependent: Especially significant for reactions involving gases.
- State function: ΔG only depends on the initial and final states of the system, not the path taken.
What is Enthalpy (ΔH)?
Enthalpy (ΔH) represents the total heat content of a system at constant pressure. It reflects the energy change during a reaction, often manifested as heat exchange with the surroundings. A negative ΔH indicates an exothermic reaction (heat is released to the surroundings), while a positive ΔH signifies an endothermic reaction (heat is absorbed from the surroundings).
Key characteristics of ΔH:
- Heat exchange: Measures the heat released or absorbed.
- Exothermic vs. Endothermic: Distinguishes between reactions that release or absorb heat.
- State function: ΔH depends only on the initial and final states.
What is Entropy (ΔS)?
Entropy (ΔS) quantifies the degree of disorder or randomness within a system. A positive ΔS indicates an increase in disorder (e.g., a solid melting into a liquid), while a negative ΔS suggests a decrease in disorder (e.g., a gas condensing into a liquid). The second law of thermodynamics states that the total entropy of an isolated system can only increase over time.
Key characteristics of ΔS:
- Disorder: A measure of randomness or chaos.
- Spontaneity: Higher entropy usually favors spontaneity.
- State function: ΔS depends only on the initial and final states.
The Relationship Between ΔG, ΔH, and ΔS: The Gibbs Free Energy Equation
The fundamental relationship between these three state functions is encapsulated in the Gibbs Free Energy equation:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy
- T is the absolute temperature (in Kelvin)
- ΔS is the change in entropy
This equation beautifully illustrates how enthalpy, entropy, and temperature interplay to determine a reaction's spontaneity. At a constant temperature, a negative ΔG is favored by a negative ΔH (exothermic reaction) and a positive ΔS (increase in disorder). However, even if ΔH is positive (endothermic), a sufficiently large and positive ΔS at a high temperature can still lead to a negative ΔG and a spontaneous reaction.
Illustrative Examples with a Table
Let's illustrate the interplay of ΔG, ΔH, and ΔS with some examples represented in a table. The table below shows hypothetical scenarios at a constant temperature of 298 K (25°C). Remember that the actual values of ΔH and ΔS depend on the specific reaction and conditions.
| Scenario | ΔH (kJ/mol) | ΔS (J/mol·K) | T (K) | ΔG (kJ/mol) | Spontaneity |
|---|---|---|---|---|---|
| 1. Exothermic, Increased Disorder | -100 | +100 | 298 | -129.4 | Spontaneous at all temperatures |
| 2. Exothermic, Decreased Disorder | -50 | -50 | 298 | -34.0 | Spontaneous at all temperatures |
| 3. Endothermic, Increased Disorder | +50 | +200 | 298 | +50 - 298(0.2) = -5.6 kJ/mol | Spontaneous at high temperatures |
| 4. Endothermic, Decreased Disorder | +100 | -100 | 298 | +129.4 | Non-spontaneous at all temperatures |
| 5. Equilibrium | 0 | 0 | 298 | 0 | At equilibrium |
Explanation of the Table:
- Scenario 1: This reaction is both exothermic (ΔH<0) and leads to an increase in disorder (ΔS>0). The negative ΔG confirms its spontaneity at all temperatures.
- Scenario 2: This reaction is exothermic but leads to a decrease in disorder. However, the magnitude of the negative ΔH outweighs the negative effect of ΔS, resulting in a spontaneous reaction.
- Scenario 3: This reaction is endothermic (ΔH>0) but leads to a significant increase in disorder (ΔS>0). At 298K, the reaction is spontaneous. At lower temperatures, it would likely be non-spontaneous.
- Scenario 4: Both endothermic and leads to decreased disorder. ΔG is positive, thus non-spontaneous at all temperatures.
- Scenario 5: At equilibrium, there is no change in Gibbs Free Energy, as the system is in balance.
This table highlights the crucial role temperature plays in determining spontaneity. For endothermic reactions (positive ΔH), a sufficiently high temperature (large TΔS) can overcome the positive enthalpy change, leading to spontaneity.
Applications of ΔG, ΔH, and ΔS
Understanding these thermodynamic parameters is crucial across various fields:
- Chemistry: Predicting reaction feasibility, equilibrium constants, and reaction rates.
- Materials Science: Designing new materials with desired properties, such as stability and reactivity.
- Biochemistry: Studying metabolic pathways, enzyme kinetics, and protein folding.
- Environmental Science: Assessing the environmental impact of chemical processes and predicting pollutant behavior.
- Engineering: Designing efficient and sustainable energy systems.
Limitations and Considerations
While extremely useful, these parameters have limitations:
- Standard conditions: Values are often calculated under standard conditions (298 K and 1 atm pressure), which may not always reflect real-world scenarios.
- Kinetics: Thermodynamics predicts spontaneity but says nothing about the reaction rate. A spontaneous reaction might be incredibly slow.
- Approximations: Calculations often involve approximations, especially for complex systems.
Conclusion
Gibbs Free Energy, Enthalpy, and Entropy are fundamental thermodynamic concepts that provide invaluable insights into the spontaneity and equilibrium of chemical and physical processes. Understanding their interrelationship through the Gibbs Free Energy equation empowers us to predict the behavior of systems under various conditions. By analyzing ΔG, ΔH, and ΔS, we can make informed decisions in diverse fields, from chemical synthesis to environmental protection. While limitations exist, these thermodynamic parameters remain essential tools in scientific inquiry and technological advancement. Further exploration into their applications and nuances will continue to expand our understanding of the natural world and enable innovations across multiple scientific disciplines.
Latest Posts
Latest Posts
-
What Group Defines Themselves Through A Rejection Of The Mainstream
Mar 25, 2025
-
Transfer Function Of An Rc Circuit
Mar 25, 2025
-
How Do You Find The Molar Mass Of A Gas
Mar 25, 2025
-
Examples Include Oils Waxes And Butters
Mar 25, 2025
-
Common Particles With Charge Of 2
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Delta G Delta H Delta S Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
