Density Of Water At Temperature Chart
Muz Play
Mar 22, 2025 · 5 min read
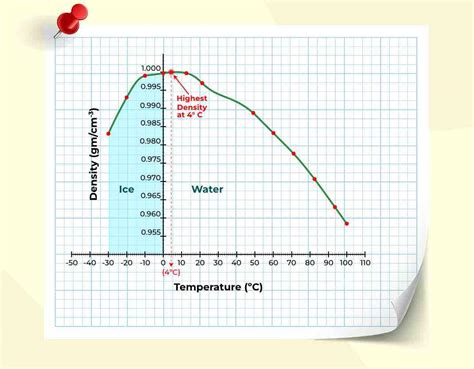
Table of Contents
Density of Water at Temperature: A Comprehensive Chart and Guide
Understanding the density of water at different temperatures is crucial across numerous scientific disciplines and practical applications. From marine biology and oceanography to industrial processes and even cooking, the relationship between water's temperature and density significantly influences behavior and outcomes. This comprehensive guide provides a detailed look at this relationship, offering a chart of water density at various temperatures, explanations of the underlying science, and discussions of its importance in various fields.
Understanding Water Density
Water density, typically expressed in kilograms per cubic meter (kg/m³) or grams per cubic centimeter (g/cm³), describes the mass of water contained within a unit volume. Unlike many substances, water exhibits an unusual density-temperature relationship.
The Anomaly of Water: Maximum Density at 4°C
Most substances become denser as their temperature decreases. However, water displays a unique anomaly. Its density increases as it cools down until it reaches its maximum density at approximately 4°C (39.2°F). Below 4°C, the density of water starts to decrease, a phenomenon crucial for aquatic life in colder climates. This unusual behavior is attributed to the hydrogen bonding within water molecules.
- Hydrogen Bonding: Water molecules are polar, meaning they have a slightly positive and slightly negative end. This polarity leads to hydrogen bonds – relatively weak attractions between the positive end of one molecule and the negative end of another. As water cools, the hydrogen bonds become more organized, causing the molecules to arrange themselves in a less dense, crystalline structure (ice).
Density Variation with Temperature: A Closer Look
The decrease in density below 4°C is why ice floats on water. This seemingly simple observation has profound ecological implications, preventing bodies of water from freezing solid and allowing aquatic life to survive even during harsh winters. Above 4°C, the density decreases gradually as temperature rises.
Density of Water at Temperature Chart
While precise values depend on factors like pressure and salinity, the following chart provides a general representation of water density at various temperatures. Remember that these are approximate values, and more precise measurements require specialized equipment and controlled conditions.
| Temperature (°C) | Density (kg/m³) | Density (g/cm³) |
|---|---|---|
| 0 | 999.84 | 0.99984 |
| 4 | 1000.00 | 1.00000 |
| 10 | 999.70 | 0.99970 |
| 15 | 999.10 | 0.99910 |
| 20 | 998.21 | 0.99821 |
| 25 | 997.05 | 0.99705 |
| 30 | 995.65 | 0.99565 |
| 35 | 994.03 | 0.99403 |
| 40 | 992.22 | 0.99222 |
| 50 | 988.04 | 0.98804 |
| 60 | 983.20 | 0.98320 |
| 70 | 977.77 | 0.97777 |
| 80 | 971.81 | 0.97181 |
| 90 | 965.32 | 0.96532 |
| 100 | 958.40 | 0.95840 |
Note: This chart assumes standard atmospheric pressure (1 atm). Changes in pressure will slightly affect water density.
Factors Affecting Water Density
Several factors beyond temperature influence water density:
- Pressure: Increasing pressure increases water density. This effect is more pronounced at greater depths in oceans.
- Salinity: Saltwater is denser than freshwater. The higher the salt concentration (salinity), the greater the water density. This salinity gradient is crucial for ocean currents.
- Dissolved Substances: The presence of dissolved substances, such as minerals and gases, can alter water density. This is relevant in various natural and industrial settings.
Applications and Importance of Understanding Water Density
Knowledge of water density at different temperatures is essential across a wide range of fields:
Oceanography and Marine Biology
- Ocean Currents: Differences in water density, driven by temperature and salinity variations, are the primary drivers of ocean currents, which play a vital role in global climate regulation and marine ecosystem health.
- Deep-Sea Exploration: Understanding water density at different depths is critical for designing and operating submersibles and other deep-sea exploration equipment.
- Marine Life Distribution: The density variations in water influence the buoyancy of marine organisms and affect their distribution and behavior.
Meteorology and Climatology
- Cloud Formation: The density of water vapor in the atmosphere is a critical factor in cloud formation and precipitation patterns.
- Weather Forecasting: Water density plays a role in atmospheric models used for weather forecasting.
Industrial Processes
- Cooling Systems: Water's density is crucial in designing and optimizing cooling systems in various industrial applications.
- Fluid Dynamics: Understanding water density is fundamental in fluid dynamics calculations and simulations used in many industrial processes.
- Chemical Engineering: In chemical reactions involving water, density is an important parameter to consider.
Food Science and Cooking
- Boiling Point: The density of water affects its boiling point, influencing cooking times and food textures.
- Density of Ingredients: The relative densities of water and other ingredients are critical in creating emulsions and suspensions in food preparation.
Environmental Science
- Water Quality Monitoring: Density measurements can be used to monitor water quality, indicating the presence of pollutants or changes in salinity.
- Pollution Control: Understanding water density is crucial in designing and implementing pollution control strategies.
Further Research and Considerations
This guide provides a basic overview of water density and its temperature dependence. For more in-depth information, consider exploring advanced topics like:
- The equation of state for seawater: This equation provides highly accurate calculations of seawater density based on temperature, pressure, and salinity.
- The impact of isotopes: The isotopic composition of water can slightly influence its density.
- Density measurements techniques: Learn about various methods used to accurately measure water density, such as pycnometry and densitometry.
Conclusion:
The relationship between water density and temperature is a complex and fascinating phenomenon with significant implications across numerous scientific disciplines and practical applications. Understanding this relationship is crucial for accurate modeling, effective design, and informed decision-making in various fields. This guide has provided a foundational understanding, and further exploration into this topic will undoubtedly reveal even more about this fundamental property of water. The information provided here serves as a solid starting point for individuals interested in learning more about this important scientific concept. Remember to consult reliable sources and scientific literature for more detailed and precise information.
Latest Posts
Latest Posts
-
What Are The Principles Of Science
Mar 23, 2025
-
Does Electronegativity Decrease Down A Group
Mar 23, 2025
-
Derive Stefans Law From Plancks Radiation Law
Mar 23, 2025
-
A Buffer Is A Substance That
Mar 23, 2025
-
Completa Las Oraciones Usando El Preterito De Ser O Ir
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Density Of Water At Temperature Chart . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
