Difference Between A Reactant And A Product
Muz Play
Mar 27, 2025 · 7 min read
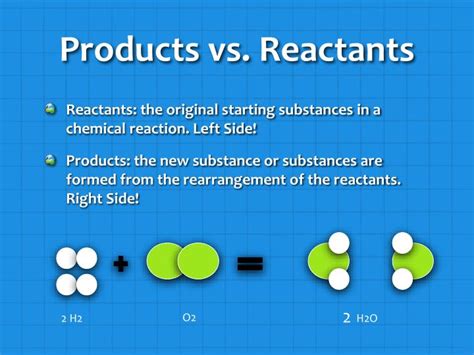
Table of Contents
The Fundamental Difference Between Reactants and Products in Chemical Reactions
Understanding the difference between reactants and products is foundational to grasping the core concepts of chemistry. While seemingly simple, a clear understanding of these terms is crucial for interpreting chemical equations, predicting reaction outcomes, and comprehending the very essence of chemical change. This article will delve deep into the distinction between reactants and products, exploring their roles in chemical reactions, providing illustrative examples, and highlighting the importance of this fundamental concept in various chemical processes.
What are Reactants?
Reactants are the starting materials in a chemical reaction. They are the substances that undergo a chemical change, transforming into new substances called products. Think of reactants as the ingredients in a recipe; they are the components that are combined to create something new. In a chemical equation, reactants are written on the left-hand side of the arrow.
Key Characteristics of Reactants:
- Undergo Transformation: Reactants are chemically altered during the reaction. Their atoms rearrange, bond, or break, leading to the formation of entirely new substances.
- Consumption: Reactants are consumed or used up during the reaction. As the reaction proceeds, the amount of reactants decreases until they are either fully consumed or reach an equilibrium state.
- Located on the Left: In chemical equations, reactants are always written on the left side of the arrow, separated by plus signs (+) if multiple reactants are involved.
- Determine the Products: The nature and quantity of the reactants significantly influence the type and amount of products formed.
What are Products?
Products are the newly formed substances resulting from a chemical reaction. They are the outcomes of the chemical transformation of the reactants. Continuing the culinary analogy, products are the finished dish – the result of combining and altering the ingredients (reactants). In a chemical equation, products are written on the right-hand side of the arrow.
Key Characteristics of Products:
- Newly Formed Substances: Products possess different chemical properties and compositions than the reactants. They are entirely new entities created through the rearrangement of atoms.
- Appearance and Formation: Products can appear in various forms, including solids (precipitates), liquids, gases, or even a mixture of states. Their formation signifies the completion of the chemical reaction.
- Located on the Right: In chemical equations, products are always written on the right side of the arrow, separated by plus signs (+) if multiple products are formed.
- Dependent on Reactants: The nature and quantity of the products are directly dependent on the reactants and the conditions of the reaction.
Illustrative Examples: Understanding Reactants and Products in Action
Let's illustrate the difference between reactants and products with several examples:
Example 1: Combustion of Methane
The combustion of methane (natural gas) is a familiar example. The balanced chemical equation is:
CH₄ (g) + 2O₂ (g) → CO₂ (g) + 2H₂O (g)
Here:
- Reactants: Methane (CH₄) and Oxygen (O₂) are the reactants. They combine to initiate the reaction.
- Products: Carbon dioxide (CO₂) and Water (H₂O) are the products formed as a result of the combustion.
Example 2: Formation of Water
The formation of water from hydrogen and oxygen is another classic example:
2H₂ (g) + O₂ (g) → 2H₂O (l)
- Reactants: Hydrogen (H₂) and Oxygen (O₂) are the reactants.
- Products: Water (H₂O) is the product.
Example 3: Neutralization Reaction
The neutralization reaction between an acid and a base is a common example in chemistry. For instance, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH):
HCl (aq) + NaOH (aq) → NaCl (aq) + H₂O (l)
- Reactants: Hydrochloric acid (HCl) and Sodium hydroxide (NaOH) are the reactants.
- Products: Sodium chloride (NaCl) and Water (H₂O) are the products.
Example 4: A More Complex Reaction – Synthesis of Ammonia
The Haber-Bosch process for the synthesis of ammonia is more complex, involving multiple reactants and products under specific conditions:
N₂ (g) + 3H₂ (g) ⇌ 2NH₃ (g)
- Reactants: Nitrogen (N₂) and Hydrogen (H₂) are the reactants. Note the double arrow (⇌), indicating a reversible reaction.
- Products: Ammonia (NH₃) is the product. The reversible nature means ammonia can also decompose back into nitrogen and hydrogen.
These examples clearly illustrate how reactants are transformed into products through a chemical change, highlighting their distinct roles in chemical reactions.
The Importance of Balancing Chemical Equations
Accurately representing chemical reactions requires balancing the chemical equation. This ensures that the number of atoms of each element remains the same on both sides of the equation – reflecting the Law of Conservation of Mass. Balancing the equation ensures that the number of atoms of each element remains consistent before and after the reaction. This is crucial because atoms are neither created nor destroyed during a chemical reaction; they are merely rearranged.
Factors Influencing Reactants and Products
Several factors can influence the outcome of a chemical reaction, affecting both the reactants and the products:
- Temperature: Changes in temperature can significantly impact reaction rates and even the products formed.
- Pressure: Pressure variations, particularly for gaseous reactants, can influence reaction equilibrium and product yields.
- Concentration: The concentrations of reactants can directly affect the rate of reaction and the amount of product formed.
- Catalyst: The presence of a catalyst can accelerate the reaction rate without being consumed itself, potentially influencing the yield of specific products.
- Solvent: The choice of solvent, especially in solution-phase reactions, can significantly impact reaction kinetics and the nature of the products.
Reactants and Products in Everyday Life
The concept of reactants and products isn't confined to the laboratory. It underpins countless processes in our daily lives:
- Cooking: Cooking involves numerous chemical reactions. The ingredients (reactants) are transformed by heat and other processes to create the finished dish (product).
- Respiration: Cellular respiration is a vital process where glucose (reactant) reacts with oxygen (reactant) to produce energy (ATP), carbon dioxide, and water (products).
- Photosynthesis: Plants convert carbon dioxide and water (reactants) into glucose (product) and oxygen (product) using sunlight as energy.
- Rusting: The rusting of iron involves a chemical reaction between iron (reactant) and oxygen (reactant) in the presence of water to form iron oxide (product), commonly known as rust.
- Combustion Engines: Combustion engines rely on the combustion of fuel (reactant) and oxygen (reactant) to produce energy to move a vehicle, with carbon dioxide and water being some of the products.
Advanced Concepts Related to Reactants and Products
For a more comprehensive understanding, consider the following advanced concepts:
- Limiting Reactants: In many reactions, one reactant is present in a smaller amount than required for complete consumption of all reactants. This reactant is the limiting reactant, determining the maximum amount of product that can be formed.
- Excess Reactants: The reactants present in larger amounts than required are called excess reactants. Some of these reactants will remain unreacted after the reaction is complete.
- Equilibrium: Many chemical reactions are reversible, reaching a state of equilibrium where the rates of the forward and reverse reactions are equal. At equilibrium, the concentrations of reactants and products remain constant.
- Reaction Kinetics: Reaction kinetics studies the rates of chemical reactions and the factors influencing them. Understanding reaction kinetics helps predict how quickly reactants are consumed and products are formed.
- Thermodynamics: Thermodynamics explores the energy changes associated with chemical reactions. It determines whether a reaction is spontaneous and can predict the equilibrium position of a reaction.
Conclusion
The distinction between reactants and products is fundamental to understanding chemical reactions. Reactants, the starting materials, undergo transformation to form products, the newly created substances. This seemingly simple concept underpins a vast array of chemical processes, from everyday occurrences to complex industrial reactions. A firm grasp of this difference, coupled with an understanding of related concepts like balancing equations, limiting reactants, and reaction kinetics, is crucial for anyone seeking to master the field of chemistry. By understanding the interplay between reactants and products, we can begin to unravel the complex world of chemical transformations and their impact on our lives.
Latest Posts
Latest Posts
-
Charging And Discharging Of Capacitor Formula
Mar 30, 2025
-
When Do You Use Henderson Hasselbalch Equation
Mar 30, 2025
-
What Happens To The Temperature During A Phase Change
Mar 30, 2025
-
A Tentative Explanation For An Event Which Can Be Testabl
Mar 30, 2025
-
How To Check If A Matrix Is Invertible
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Difference Between A Reactant And A Product . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
