Difference Between E1 And E2 Reaction
Muz Play
Apr 01, 2025 · 6 min read
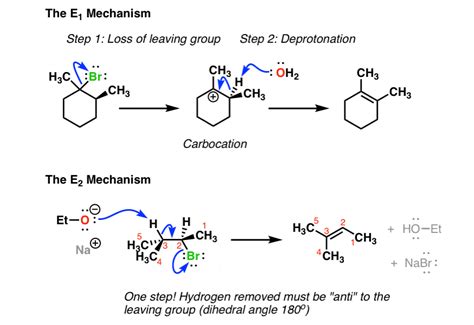
Table of Contents
Unveiling the Distinctions: E1 vs. E2 Elimination Reactions
Organic chemistry often presents a fascinating landscape of reactions, and among the most crucial are elimination reactions. These reactions involve the removal of atoms or groups from a molecule, leading to the formation of a double or triple bond. Two prominent elimination reactions are E1 and E2, each characterized by distinct mechanisms, reaction conditions, and product distributions. Understanding the nuances between these two reactions is crucial for mastering organic chemistry. This comprehensive guide delves deep into the differences between E1 and E2 reactions, highlighting key features and providing practical examples.
Defining the Players: E1 and E2 Elimination Reactions
Both E1 and E2 reactions are elimination reactions, resulting in the removal of a leaving group and a proton (H⁺) from adjacent carbon atoms. However, their mechanisms diverge significantly, leading to different stereochemical outcomes and reaction kinetics.
E1 Reaction: A UniMolecular Elimination
E1, or unimolecular elimination, is a two-step process involving the formation of a carbocation intermediate. This reaction typically occurs with tertiary (3°) alkyl halides and sometimes with secondary (2°) alkyl halides, though rarely with primary (1°) alkyl halides.
Step 1: Ionization
The C-X bond (where X is a leaving group like halide or tosylate) heterolytically cleaves, resulting in the formation of a carbocation and a leaving group anion. This step is the rate-determining step, hence the unimolecular designation.
Step 2: Deprotonation
A base (often a weak base like water or a solvent molecule) abstracts a proton (β-hydrogen) from a carbon atom adjacent to the carbocation. This results in the formation of a double bond (alkene) and a conjugate acid of the base.
E2 Reaction: A Bimolecular Elimination
E2, or bimolecular elimination, is a concerted one-step process where the removal of the leaving group and the proton occur simultaneously. This reaction is favored by strong bases like hydroxide (OH⁻), alkoxide (RO⁻), and tertiary amines. Primary and secondary alkyl halides readily undergo E2 reactions. Tertiary alkyl halides can also undergo E2 reactions, but steric hindrance can sometimes favor E1.
Concerted Mechanism:
In the E2 mechanism, the base attacks the β-hydrogen while the leaving group departs, creating a new π-bond in a single step. The transition state involves both the base, the substrate, and the leaving group.
Key Differences Between E1 and E2 Reactions
The following table summarizes the key distinctions between E1 and E2 reactions:
| Feature | E1 Reaction | E2 Reaction |
|---|---|---|
| Mechanism | Two-step (ionization followed by deprotonation) | Concerted (one-step) |
| Rate Law | Rate = k[substrate] | Rate = k[substrate][base] |
| Order of Reaction | First-order | Second-order |
| Substrate | Tertiary > Secondary > Primary (rare) | Primary, Secondary, and Tertiary (though steric hindrance can affect E2 with tertiary substrates) |
| Base | Weak base (e.g., water, alcohol) | Strong base (e.g., hydroxide, alkoxide) |
| Stereochemistry | Non-stereospecific (mixture of stereoisomers possible due to carbocation intermediate) | Stereoselective (anti-periplanar preferred) |
| Carbocation Intermediate | Forms | Does not form |
| Reaction Conditions | Often higher temperatures and polar protic solvents | Can occur at lower temperatures, variety of solvents |
| Rearrangements | Can occur (carbocation rearrangements) | Does not occur |
Factors Influencing E1 vs. E2 Reactions
Several factors influence whether a reaction proceeds via an E1 or E2 mechanism. These include:
- Substrate Structure: Tertiary substrates strongly favor E1, while primary substrates favor E2. Secondary substrates can undergo both E1 and E2 depending on the reaction conditions.
- Base Strength: Strong bases promote E2, while weak bases favor E1.
- Solvent: Polar protic solvents stabilize the carbocation intermediate in E1, while polar aprotic solvents are better for E2 reactions as they solvate the cation but not the anion, keeping the base reactive.
- Temperature: Higher temperatures usually favor E1 reactions due to the higher activation energy of the first step.
- Leaving Group Ability: A good leaving group is essential for both mechanisms. Common examples include halides (I⁻ > Br⁻ > Cl⁻ > F⁻) and tosylate (OTs⁻).
Stereochemistry: A Tale of Two Mechanisms
The stereochemistry of the products differs significantly between E1 and E2 reactions.
E1 Stereochemistry: Non-stereospecific
The formation of a planar carbocation intermediate in E1 reactions leads to a non-stereospecific outcome. Deprotonation can occur from either side of the carbocation, resulting in a mixture of stereoisomers (cis and trans alkenes). The ratio of cis and trans isomers depends on steric factors and the stability of each isomer.
E2 Stereochemistry: Stereoselective
E2 reactions are stereoselective, preferring an anti-periplanar geometry. This means the β-hydrogen and the leaving group must be on opposite sides of the molecule and in the same plane. This arrangement allows for optimal orbital overlap in the transition state, leading to the formation of the alkene. If the anti-periplanar conformation is not possible (e.g., in a cis-disubstituted cyclohexane), the reaction may be slower or proceed via a syn-periplanar geometry (less favored).
Practical Examples and Applications
Let's examine some examples to solidify our understanding:
Example 1: E1 Reaction
The reaction of tert-butyl bromide with ethanol at elevated temperatures favors E1 elimination, yielding a mixture of 2-methylpropene (major product) and possibly a small amount of its isomer.
Example 2: E2 Reaction
The reaction of 2-bromobutane with potassium tert-butoxide (strong base) in tert-butanol (polar aprotic solvent) favors E2 elimination, predominantly forming 2-butene. The anti-periplanar conformation dictates the stereochemistry of the alkene formed.
Distinguishing E1 and E2 Reactions Experimentally
Several experimental techniques can help distinguish between E1 and E2 reactions:
- Kinetic Studies: Measuring the reaction rate with varying substrate and base concentrations can determine the reaction order, providing clues about the mechanism.
- Product Analysis: Analyzing the stereochemistry and ratio of products can indicate whether a carbocation intermediate was involved (E1) or an anti-periplanar transition state (E2).
- Isotope Labeling: Using deuterium labeling can provide insights into the mechanism by tracking the movement of protons.
Conclusion: Mastering the Nuances of Elimination Reactions
Understanding the differences between E1 and E2 reactions is pivotal for success in organic chemistry. The choice between these mechanisms depends on a variety of factors, including the substrate structure, base strength, solvent, and temperature. By carefully considering these factors, we can predict the outcome of elimination reactions and design synthetic strategies effectively. The ability to distinguish between E1 and E2 reactions through experimental techniques allows for a deeper understanding of reaction mechanisms and the fine details of organic chemistry. While this guide provides a comprehensive overview, continued exploration and hands-on experience are crucial for mastering this fundamental aspect of organic chemistry. Remember to consider all factors mentioned above when predicting the outcome of an elimination reaction. This will enable you to effectively apply this knowledge in advanced organic chemistry contexts.
Latest Posts
Latest Posts
-
Activation Energy For The Forward Reaction
Apr 02, 2025
-
Base Excision Repair Vs Mismatch Repair
Apr 02, 2025
-
Is Argon Metal Nonmetal Or Metalloid
Apr 02, 2025
-
What Is A Limiting Amino Acid In A Protein
Apr 02, 2025
-
Under What Conditions Are Gases Most Likely To Behave Ideally
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Difference Between E1 And E2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
