Difference Between Electrolytic And Voltaic Cell
Muz Play
Mar 24, 2025 · 7 min read
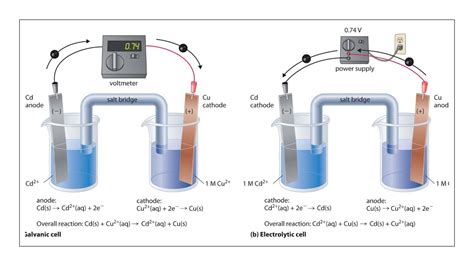
Table of Contents
Delving Deep into the Differences: Electrolytic vs. Voltaic Cells
Electrolytic and voltaic cells, while both involving electrochemical processes, operate under fundamentally different principles. Understanding their distinctions is crucial for anyone studying chemistry, electrochemistry, or related fields. This comprehensive guide will dissect the core differences, exploring their mechanisms, applications, and key characteristics. We'll delve into the nuances to provide you with a thorough understanding of these essential electrochemical systems.
Core Differences: A Quick Overview
Before diving into the intricacies, let's establish the fundamental differences between electrolytic and voltaic cells:
| Feature | Electrolytic Cell | Voltaic (Galvanic) Cell |
|---|---|---|
| Energy Source | External power source (e.g., battery) | Spontaneous chemical reaction |
| Process | Non-spontaneous redox reaction; electrolysis | Spontaneous redox reaction; cell potential > 0 |
| Electron Flow | From cathode to anode (external circuit) | From anode to cathode (external circuit) |
| Purpose | Drive non-spontaneous reactions; produce chemicals | Generate electricity from chemical reactions |
| ΔG | Positive (requires energy input) | Negative (releases energy) |
| Ecell | Negative (non-spontaneous) | Positive (spontaneous) |
Electrolytic Cells: Forcing Non-Spontaneous Reactions
Electrolytic cells are electrochemical cells that use an external electric current to drive a non-spontaneous redox reaction. Think of it as forcing a chemical reaction to occur in a direction it wouldn't naturally go. This process, known as electrolysis, is crucial in various industrial applications.
The Mechanism of Electrolysis
The process starts with an external power source (like a battery) connected to two electrodes immersed in an electrolyte solution. The electrolyte is a substance that conducts electricity through the movement of ions.
-
Anode: The anode is the positive electrode. Here, oxidation occurs – electrons are lost by a substance. This substance is often a metal or a halide ion.
-
Cathode: The cathode is the negative electrode. Here, reduction occurs – electrons are gained by a substance. This substance could be a metal cation, a hydrogen ion (H+), or another suitable species.
-
Electron Flow: The external power source forces electrons to flow from the cathode to the anode, against the natural flow of electrons. This is why energy input is required.
-
Ion Movement: Simultaneously, ions in the electrolyte solution migrate to neutralize the charge buildup at the electrodes. Anions (negatively charged ions) move towards the anode, and cations (positively charged ions) move towards the cathode.
Applications of Electrolytic Cells
Electrolytic cells are workhorses in various industries:
-
Electroplating: Coating a metal object with a thin layer of another metal, enhancing its appearance, corrosion resistance, or functionality. This is commonly used in jewelry making, automotive parts, and other applications.
-
Electrorefining: Producing high-purity metals from impure samples. The impure metal is used as the anode, and pure metal is deposited at the cathode.
-
Electrometallurgy: Extracting metals from their ores. For example, aluminum is primarily produced through the Hall-Héroult process, which uses an electrolytic cell.
-
Chlor-alkali process: Producing chlorine gas (Cl2), hydrogen gas (H2), and sodium hydroxide (NaOH) by electrolyzing brine (sodium chloride solution). This is a crucial process in the chemical industry.
Voltaic (Galvanic) Cells: Harnessing Spontaneous Reactions
In contrast to electrolytic cells, voltaic (also called galvanic) cells harness the energy released from a spontaneous redox reaction to generate electricity. These cells are the foundation of batteries we use in everyday life.
The Mechanism of Spontaneous Reactions
Voltaic cells consist of two half-cells, each containing an electrode and an electrolyte. The two half-cells are connected by a salt bridge, which allows the flow of ions to maintain electrical neutrality.
-
Anode: The anode is the electrode where oxidation occurs. The substance losing electrons has a higher reduction potential than the substance at the cathode.
-
Cathode: The cathode is the electrode where reduction occurs. The substance gaining electrons has a lower reduction potential than the substance at the anode.
-
Electron Flow: Electrons flow spontaneously from the anode (where oxidation occurs and electrons are released) to the cathode (where reduction occurs and electrons are consumed) through an external circuit. This flow of electrons constitutes the electric current.
-
Salt Bridge: The salt bridge completes the circuit by allowing the flow of ions between the two half-cells. This prevents the buildup of charge that would otherwise stop the electron flow.
Applications of Voltaic Cells
Voltaic cells power a wide range of devices and systems:
-
Batteries: From small button cells in watches to large car batteries, voltaic cells are the backbone of portable power sources. Different battery types utilize various combinations of electrodes and electrolytes to achieve different characteristics (voltage, capacity, lifespan).
-
Fuel Cells: These cells convert the chemical energy of a fuel (often hydrogen) and an oxidant (often oxygen) directly into electricity. Fuel cells are gaining traction as a clean energy source.
-
Corrosion Prevention: By carefully selecting materials and designing voltaic cells, one can protect metals from corrosion. This often involves using sacrificial anodes (like zinc) to protect a more valuable metal (like iron).
Key Differences Summarized: A Comparative Table
To solidify our understanding, let's recap the key differences in a concise table:
| Feature | Electrolytic Cell | Voltaic (Galvanic) Cell |
|---|---|---|
| Spontaneity | Non-spontaneous | Spontaneous |
| Energy Change | ΔG > 0 (requires energy) | ΔG < 0 (releases energy) |
| Cell Potential (Ecell) | Ecell < 0 | Ecell > 0 |
| Electron Flow | Cathode to anode (external circuit) | Anode to cathode (external circuit) |
| Purpose | Drive non-spontaneous reactions; produce chemicals | Generate electricity from chemical reactions |
| External Power | Required | Not required |
| Electrode Reactions | Oxidation at anode, reduction at cathode | Oxidation at anode, reduction at cathode |
Practical Applications: Real-World Examples
Let's explore some real-world examples to further illuminate the differences between electrolytic and voltaic cells:
Electrolytic Cell Example: The production of aluminum through the Hall-Héroult process. This process requires a significant amount of electrical energy to extract aluminum from its ore (alumina). The alumina is dissolved in molten cryolite, and an external current drives the reduction of aluminum ions (Al3+) to aluminum metal at the cathode.
Voltaic Cell Example: A common alkaline battery. This battery uses a zinc anode and a manganese dioxide cathode, separated by an alkaline electrolyte. The spontaneous chemical reaction between zinc and manganese dioxide produces an electric current that powers various devices.
Beyond the Basics: Advanced Concepts
The differences extend beyond the fundamental principles. Considerations such as electrode potential, overpotential, and the Nernst equation offer a deeper understanding of the intricate processes at play.
Electrode Potential and the Nernst Equation
The electrode potential (reduction potential) of a half-cell indicates its tendency to undergo reduction. The difference in reduction potentials between the two half-cells in a voltaic cell determines the cell potential (Ecell), which dictates the voltage generated. The Nernst equation allows us to calculate the cell potential under non-standard conditions (concentrations, pressures).
Overpotential and Efficiency
Overpotential is the extra voltage required beyond the theoretical cell potential to drive a reaction at a reasonable rate. In electrolytic cells, overpotential increases the energy consumption, reducing the efficiency of the process. In voltaic cells, overpotential reduces the voltage output.
Conclusion: A Powerful Dualism
Electrolytic and voltaic cells are cornerstones of electrochemistry, representing opposing yet complementary processes. Electrolytic cells are essential for driving non-spontaneous reactions, leading to crucial industrial applications. Voltaic cells, on the other hand, offer a convenient way to harness the energy released from spontaneous reactions, powering countless devices in our daily lives. Understanding the fundamental differences and the intricate details of these electrochemical systems is pivotal for appreciating their significance in diverse scientific and technological fields. Further exploration into the specifics of different cell types and their applications will provide even deeper insights into the fascinating world of electrochemistry.
Latest Posts
Latest Posts
-
What Is The Period Of Oscillation
Mar 25, 2025
-
Adding And Subtracting Sig Figs Practice
Mar 25, 2025
-
Which Applies To The Collision Theory
Mar 25, 2025
-
How To Find The Basis Of A Matrix
Mar 25, 2025
-
Atoms That Gain Electrons Are Called
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Electrolytic And Voltaic Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
