Which Applies To The Collision Theory
Muz Play
Mar 25, 2025 · 5 min read
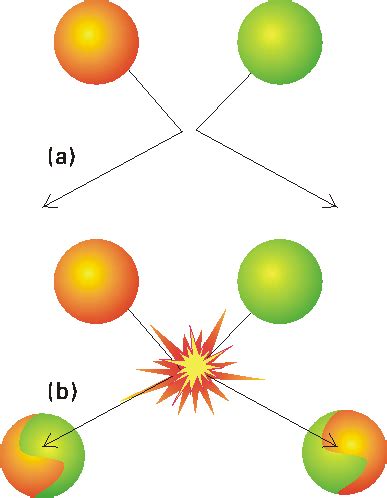
Table of Contents
Which Factors Apply to the Collision Theory? A Deep Dive into Reaction Rates
The collision theory is a cornerstone of chemical kinetics, offering a simple yet powerful model to explain the rates of chemical reactions. It posits that for a reaction to occur, reactant particles must collide with sufficient energy and proper orientation. This seemingly straightforward statement, however, encompasses a complex interplay of factors that significantly influence reaction rates. This article will delve into the specifics, exploring which factors apply to the collision theory and how they affect the speed at which reactions proceed.
The Fundamental Principles of Collision Theory
Before examining the factors, let's reiterate the core tenets of collision theory:
- Effective Collisions: Reactions only happen when reactant molecules collide with enough energy (activation energy) to break existing bonds and form new ones. This isn't just any collision; it needs to be an effective collision.
- Orientation: The orientation of colliding molecules matters. Molecules must collide in a specific way for bonds to break and reform correctly. If the orientation is unfavorable, even a high-energy collision might not lead to a reaction.
- Frequency of Collisions: The rate of a reaction is directly proportional to the frequency of collisions between reactant molecules. More collisions generally mean a faster reaction.
Factors Affecting Collision Frequency and Effectiveness
Several factors influence both the frequency and effectiveness of collisions, directly impacting the reaction rate:
1. Concentration of Reactants
Higher concentrations lead to more frequent collisions. Imagine a crowded room versus an empty one. In a crowded room (high concentration), people (reactant molecules) are more likely to bump into each other. Similarly, increasing the concentration of reactants increases the probability of successful collisions and thus accelerates the reaction rate. This relationship is often described mathematically through rate laws.
2. Temperature
Higher temperatures increase both the frequency and effectiveness of collisions. Temperature is directly related to the kinetic energy of molecules. At higher temperatures, molecules move faster, leading to:
- Increased Collision Frequency: Faster-moving molecules collide more frequently.
- Increased Effective Collisions: A higher proportion of collisions possess the necessary activation energy to overcome the energy barrier for the reaction to proceed. The Boltzmann distribution illustrates this: a higher temperature shifts the distribution towards higher energies, resulting in a larger fraction of molecules exceeding the activation energy.
3. Surface Area of Reactants
This factor primarily applies to heterogeneous reactions (reactions involving reactants in different phases, like a solid reacting with a liquid or gas).
Increased surface area provides more contact points for collisions. Consider a reaction between a solid and a liquid. A powdered solid (high surface area) will react much faster than a solid lump (low surface area) because the increased surface area allows more molecules of the liquid to come into contact with the solid, leading to a greater number of collisions.
4. Catalysts
Catalysts dramatically increase the reaction rate without being consumed in the process. They achieve this by:
- Lowering the Activation Energy: Catalysts provide an alternative reaction pathway with a lower activation energy. This means that a larger fraction of collisions will have sufficient energy to lead to a reaction, even at lower temperatures.
- Providing a More Favorable Orientation: Catalysts can bring reactant molecules together in a more favorable orientation, increasing the probability of an effective collision.
5. Pressure (for gaseous reactions)
Increased pressure for gaseous reactions increases both collision frequency and concentration. For gaseous reactants, increasing the pressure compresses the gas, leading to:
- Higher Concentration: More molecules are packed into the same volume, increasing the probability of collisions.
- Higher Collision Frequency: The molecules are closer together and moving faster due to increased intermolecular forces, resulting in more frequent collisions.
The Role of Activation Energy and the Arrhenius Equation
Activation energy (Ea) is the minimum energy required for a collision to be effective. It represents the energy barrier that reactant molecules must overcome to transform into products. The Arrhenius equation mathematically describes the relationship between the rate constant (k), temperature (T), activation energy (Ea), and the frequency factor (A):
k = A * e^(-Ea/RT)
Where:
- k: Rate constant
- A: Frequency factor (related to the frequency of collisions and the orientation factor)
- Ea: Activation energy
- R: Ideal gas constant
- T: Temperature in Kelvin
This equation highlights the exponential dependence of the rate constant on activation energy and temperature. A lower activation energy or a higher temperature significantly increases the rate constant and therefore the reaction rate.
Beyond the Basics: More nuanced considerations
While the factors above provide a solid foundation, a deeper understanding requires considering more nuanced aspects:
-
Steric Factor: The steric factor (P) accounts for the probability that colliding molecules have the correct orientation for a reaction to occur. It's often less than 1, reflecting the fact that only a fraction of collisions have the ideal geometry. This factor is incorporated into the Arrhenius equation's frequency factor (A).
-
Molecular Complexity: The complexity of molecules influences the probability of successful collisions. Larger, more complex molecules may require more specific orientations for a reaction to occur, leading to a lower steric factor.
-
Solvent Effects (for reactions in solution): The solvent can influence the reaction rate through various mechanisms, including solvation of reactants, altering the activation energy, and affecting collision frequency through viscosity.
-
Quantum Tunneling: At very low temperatures, quantum mechanical effects allow some molecules to "tunnel" through the activation energy barrier, even if they lack the required classical energy. This effect is more significant for lighter particles.
Applying Collision Theory to Real-World Examples
Understanding collision theory is crucial in numerous applications:
-
Industrial Chemistry: Optimizing reaction conditions (temperature, pressure, concentration, catalysts) to maximize product yield and reaction rate is essential in industrial processes.
-
Environmental Science: Understanding reaction rates helps predict the fate of pollutants in the environment and design effective remediation strategies.
-
Biological Systems: Enzyme-catalyzed reactions are fundamental to life, and collision theory provides insight into the efficiency of these biological catalysts.
-
Materials Science: Controlling reaction rates is vital in the synthesis of new materials with desired properties.
Conclusion: A Holistic View of Reaction Rates
Collision theory provides a robust framework for understanding the factors governing chemical reaction rates. While the basic principles focus on collision frequency and effectiveness, a deeper understanding requires considering nuanced aspects like activation energy, steric factors, and environmental influences. By comprehensively analyzing these factors, scientists and engineers can design and optimize chemical processes, contributing significantly to various fields of study and applications. The ongoing research in collision theory continues to refine our understanding of chemical reactions, revealing the intricate dance of molecules that drives the world around us.
Latest Posts
Latest Posts
-
Titration Curve Of Hcl And Naoh
Mar 26, 2025
-
List Three Physical Properties Of Water
Mar 26, 2025
-
When A Substance In A Reaction Is Oxidized It
Mar 26, 2025
-
What Happens To Electrons In Metallic Bonding
Mar 26, 2025
-
Label The Types Of Intercellular Junctions
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which Applies To The Collision Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
