Difference Between Inter And Intramolecular Forces
Muz Play
Mar 30, 2025 · 6 min read
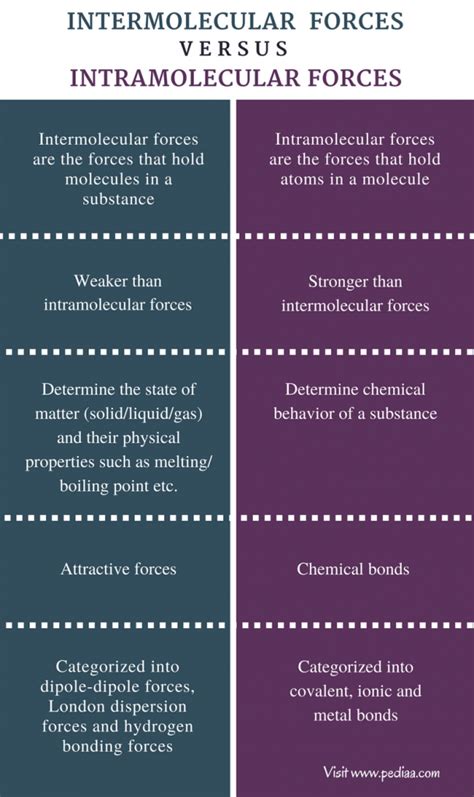
Table of Contents
Delving Deep into the Differences: Intermolecular vs. Intramolecular Forces
Understanding the fundamental forces that govern the behavior of matter is crucial in chemistry. Two key categories of forces dominate this realm: intermolecular forces and intramolecular forces. While both involve interactions between atoms, their nature and strength differ significantly, leading to vastly different consequences for the properties of substances. This comprehensive guide will illuminate the differences between these forces, exploring their individual characteristics and illustrating their impacts on the macroscopic world around us.
Intramolecular Forces: The Bonds That Hold Molecules Together
Intramolecular forces are the forces within a molecule that hold atoms together. These are the strong forces that define the molecule's structure and determine its chemical properties. They are responsible for the formation of chemical bonds, which are essentially electrostatic attractions between atoms. There are three primary types of intramolecular forces:
1. Ionic Bonds: Electrostatic Attraction at its Finest
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom (typically a metal) loses one or more electrons to another atom (typically a non-metal), forming a cation (positive ion) and an anion (negative ion), respectively. The strong Coulombic attraction between these ions creates a stable ionic compound. Examples include sodium chloride (NaCl), where sodium loses an electron to chlorine, and magnesium oxide (MgO). These bonds are characterized by high melting and boiling points due to the significant electrostatic forces involved.
2. Covalent Bonds: Sharing is Caring (Electrons, that is)
Covalent bonds are formed when atoms share electrons to achieve a stable electron configuration, often resembling a noble gas configuration. This sharing occurs between non-metal atoms. The shared electrons are attracted to the nuclei of both atoms, creating a stable bond. The strength of covalent bonds varies depending on factors like the electronegativity difference between the atoms involved. Examples include the bonds within water (H₂O), methane (CH₄), and diamond (C). Covalent compounds exhibit a wide range of melting and boiling points, depending on the strength of the covalent bonds and the presence of intermolecular forces.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds occur in metals and involve the delocalization of valence electrons. These electrons are not associated with any particular atom but are free to move throughout the metal lattice. This "sea" of electrons holds the positively charged metal ions together, resulting in high electrical and thermal conductivity, malleability, and ductility characteristic of metals. Examples include the bonds within copper (Cu), iron (Fe), and gold (Au).
Intermolecular Forces: The Subtle Interactions Shaping Matter's Properties
Intermolecular forces, unlike intramolecular forces, are forces of attraction between different molecules. These forces are generally much weaker than intramolecular forces but play a crucial role in determining the physical properties of substances, such as melting points, boiling points, viscosity, and solubility. Several types of intermolecular forces exist:
1. Van der Waals Forces: Weak but Widespread Interactions
Van der Waals forces are a collective term encompassing several weak intermolecular forces. They arise from temporary fluctuations in electron distribution around atoms and molecules. These fluctuations create temporary dipoles, which induce dipoles in neighboring molecules, leading to weak attractive forces. There are three main types of Van der Waals forces:
-
London Dispersion Forces (LDFs): These are the weakest type of intermolecular force and are present in all molecules, regardless of their polarity. They arise from instantaneous dipole-induced dipole interactions. The strength of LDFs increases with the size and shape of the molecule; larger molecules with greater surface area have stronger LDFs.
-
Dipole-Dipole Forces: These forces occur between polar molecules, which have a permanent dipole moment due to unequal sharing of electrons. The positive end of one polar molecule is attracted to the negative end of another. These are stronger than LDFs but weaker than hydrogen bonds.
-
Ion-Dipole Forces: These forces occur between ions and polar molecules. The charged ion is attracted to the oppositely charged end of the polar molecule. These are stronger than dipole-dipole forces.
2. Hydrogen Bonds: A Special Case of Dipole-Dipole Interaction
Hydrogen bonds are a special type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) and is attracted to another electronegative atom in a nearby molecule. Hydrogen bonds are significantly stronger than other dipole-dipole interactions because of the high electronegativity difference and the small size of the hydrogen atom, leading to a strong electrostatic attraction. Hydrogen bonding is responsible for the unique properties of water, such as its high boiling point and surface tension.
Comparing Intramolecular and Intermolecular Forces: A Head-to-Head Comparison
| Feature | Intramolecular Forces | Intermolecular Forces |
|---|---|---|
| Strength | Strong | Weak |
| Type of Interaction | Within a molecule | Between molecules |
| Bonding | Forms chemical bonds | Does not form chemical bonds |
| Effect on Properties | Determines chemical properties | Determines physical properties |
| Examples | Ionic, covalent, metallic bonds | Van der Waals forces, hydrogen bonds |
| Energy Involved | High energy | Low energy |
| Melting/Boiling Points | Generally high (except for some covalent compounds) | Generally low (except for substances with strong hydrogen bonding) |
The Impact of Intermolecular and Intramolecular Forces on Physical Properties
The interplay between intramolecular and intermolecular forces profoundly affects the physical properties of substances. Consider the following examples:
-
Boiling Point: Substances with strong intermolecular forces (e.g., those with hydrogen bonds) have higher boiling points because more energy is required to overcome the attractive forces between molecules and transition to the gaseous phase. Conversely, substances with weak intermolecular forces (e.g., those with only LDFs) have lower boiling points.
-
Melting Point: Similar to boiling point, the strength of intermolecular forces directly impacts the melting point. Substances with strong intermolecular forces require more energy to overcome the attractive forces in the solid state and transition to the liquid state.
-
Solubility: The solubility of a substance depends on the balance of intermolecular forces between the solute and the solvent. "Like dissolves like" is a common adage, meaning polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes. This is because similar intermolecular forces facilitate interaction and dissolution.
-
Viscosity: The viscosity, or resistance to flow, of a liquid is influenced by the strength of intermolecular forces. Liquids with strong intermolecular forces tend to be more viscous.
-
Surface Tension: Surface tension is a measure of the cohesive forces between molecules at the surface of a liquid. Stronger intermolecular forces lead to higher surface tension.
Conclusion: A Symphony of Forces
Intramolecular and intermolecular forces are two distinct but interconnected concepts. Intramolecular forces are the "glue" that holds molecules together, dictating chemical properties, while intermolecular forces determine the physical properties of substances. Understanding the differences and relative strengths of these forces is essential for comprehending the behavior of matter and predicting the properties of various substances. The intricate balance between these forces shapes the world around us, from the solid structure of a crystal to the fluidity of water and the volatility of gases. The interplay between them is a complex but fascinating dance that dictates the properties of the materials we encounter every day. Further exploration into these forces unveils the fundamental principles governing chemistry and material science.
Latest Posts
Latest Posts
-
Van Der Waals Equation Constants A And B
Apr 01, 2025
-
What Does A Sigma Bond Look Like
Apr 01, 2025
-
What Are The Possible Offspring Genotypes
Apr 01, 2025
-
How To Calculate Voltage Of A Cell
Apr 01, 2025
-
What Are The Rights And Duties Of A Citizen
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Inter And Intramolecular Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
