What Does A Sigma Bond Look Like
Muz Play
Apr 01, 2025 · 6 min read
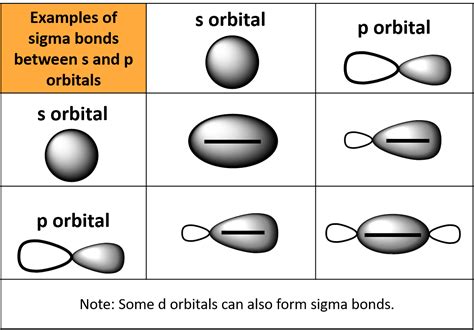
Table of Contents
What Does a Sigma Bond Look like? A Deep Dive into Chemical Bonding
Understanding chemical bonds is fundamental to grasping the behavior of matter. Among the various types of chemical bonds, the sigma (σ) bond holds a special place, forming the backbone of countless molecules. But what does a sigma bond actually look like? This article will delve into the visual representation, formation, properties, and significance of sigma bonds, going beyond simple textbook diagrams to provide a comprehensive understanding.
The Visual Representation: Beyond the Simple Overlap
Textbooks often depict a sigma bond as a simple line connecting two atoms. While convenient, this representation is significantly simplified and doesn't convey the complex three-dimensional nature of the bond. A more accurate visual depends on the type of atoms involved and the bonding orbitals.
Head-on Overlap of Atomic Orbitals
The defining characteristic of a sigma bond is the head-on overlap of atomic orbitals. Imagine two balloons touching each other directly at their tips – this is analogous to the overlap of s orbitals or the head-on overlap of p orbitals. This head-on overlap leads to a region of high electron density directly between the two bonded nuclei.
This region of high electron density is crucial. It creates a strong electrostatic attraction between the positively charged nuclei and the negatively charged electrons, holding the atoms together.
Visualizing s-s Sigma Bonds
The simplest example is the sigma bond formed between two hydrogen atoms (H₂). Each hydrogen atom possesses a single 1s orbital containing one electron. When they approach each other, these 1s orbitals overlap directly, forming a cylindrical region of electron density centered along the internuclear axis. This cylindrical symmetry is a key feature of sigma bonds.
Visualizing s-p Sigma Bonds
Consider the hydrogen fluoride (HF) molecule. Hydrogen contributes a spherical 1s orbital, while fluorine contributes a dumbbell-shaped 2p orbital. The sigma bond forms through the overlap of the hydrogen 1s orbital with one of the fluorine 2p orbitals. The overlap isn't perfectly symmetrical, but it's still head-on, resulting in a region of increased electron density concentrated between the two nuclei.
Visualizing p-p Sigma Bonds
In molecules like fluorine (F₂), each fluorine atom contributes a half-filled 2p orbital. The sigma bond is formed by the direct head-on overlap of these 2p orbitals. Again, this leads to a cylindrical electron density region along the internuclear axis.
Formation of Sigma Bonds: Orbital Hybridization and Molecular Geometry
The formation of sigma bonds is intricately linked to the concept of orbital hybridization. This is particularly crucial when considering atoms with multiple bonds or complex molecular geometries.
sp Hybridization
When an atom forms a triple bond (one sigma and two pi bonds), its s and p orbitals hybridize to form two sp hybrid orbitals. These hybrid orbitals are linear and are involved in forming one sigma bond each, in opposite directions, with the remaining p-orbitals forming pi bonds. Ethene (C₂H₂) is a great example.
sp² Hybridization
In molecules with double bonds (one sigma and one pi bond), the s orbital and two p orbitals hybridize to form three sp² hybrid orbitals. These hybrid orbitals are arranged in a trigonal planar geometry, with 120° angles between them. One of the sp² hybrids is involved in forming a sigma bond, while the unhybridized p orbital participates in the formation of the pi bond. Ethene (C₂H₄) is a classic example, showing this type of bonding.
sp³ Hybridization
In molecules with single bonds, the s orbital and three p orbitals hybridize to form four sp³ hybrid orbitals. These are arranged in a tetrahedral geometry with bond angles of approximately 109.5°. Each sp³ hybrid orbital is involved in forming a sigma bond. Methane (CH₄) is a textbook example of sp³ hybridization.
Properties of Sigma Bonds: Strength, Rotational Freedom, and Reactivity
Sigma bonds possess unique properties that significantly influence the properties of molecules.
Bond Strength
Sigma bonds are generally stronger than pi bonds. This is because the head-on overlap of atomic orbitals leads to a higher electron density concentrated directly between the nuclei, resulting in a stronger electrostatic attraction.
Rotational Freedom
Unlike pi bonds, sigma bonds allow for free rotation around the internuclear axis. This is because the cylindrical symmetry of the electron cloud doesn't hinder rotation. This rotational freedom plays a significant role in the conformational analysis of molecules.
Reactivity
Sigma bonds are generally less reactive than pi bonds. The high electron density between the nuclei makes them relatively stable and less susceptible to electrophilic or nucleophilic attacks. However, they can participate in reactions, particularly when stronger bonds are formed.
Sigma Bonds vs. Pi Bonds: A Key Distinction
It's crucial to understand the difference between sigma (σ) and pi (π) bonds. Both are types of covalent bonds, but they differ in their formation and properties.
-
Sigma Bonds: Formed by head-on overlap of atomic orbitals, resulting in a cylindrical electron density distribution around the internuclear axis. They are stronger and allow for free rotation.
-
Pi Bonds: Formed by sideways overlap of p orbitals, resulting in electron density above and below the internuclear axis. They are weaker and don't allow for free rotation. They always occur in conjunction with a sigma bond.
Significance of Sigma Bonds in Organic and Inorganic Chemistry
Sigma bonds form the structural backbone of countless organic and inorganic molecules. Their strength and stability are essential for the existence and function of many biological molecules.
Organic Chemistry
In organic chemistry, carbon's ability to form four sigma bonds is fundamental. This ability allows carbon to form long chains, branched structures, and rings, leading to the vast diversity of organic compounds. The properties of organic molecules, including their reactivity and physical properties, are heavily influenced by the sigma bonds that hold them together.
Inorganic Chemistry
Sigma bonds play a vital role in inorganic chemistry as well. Many inorganic compounds, particularly those containing elements from the main groups, are held together by sigma bonds. The understanding of sigma bonds is crucial for analyzing the structure and reactivity of these compounds.
Advanced Concepts: Delocalized Sigma Bonds and Hyperconjugation
Beyond the basic understanding, there are more nuanced concepts related to sigma bonds:
Delocalized Sigma Bonds
In certain systems, particularly those with extensive conjugation, sigma bonds can participate in delocalization. This means that the electron density in the sigma bond isn't confined strictly to the two atoms involved but is spread out over a larger region. This delocalization can contribute to enhanced stability and unique reactivity patterns.
Hyperconjugation
Hyperconjugation is a stabilizing interaction between a sigma bond and an adjacent empty or partially filled p-orbital. This interaction involves the donation of electron density from the sigma bond to the p-orbital, increasing the stability of the molecule. Hyperconjugation plays an important role in determining the stability and reactivity of carbocations and other organic molecules.
Conclusion: A Foundation of Chemical Structure and Reactivity
The sigma bond, though often represented simply, is a complex and crucial aspect of chemical bonding. Its unique characteristics, including its head-on overlap, strength, rotational freedom, and participation in advanced phenomena like delocalization and hyperconjugation, have profound implications for the structure, properties, and reactivity of molecules in all areas of chemistry. Understanding the "what" and "how" of a sigma bond is essential for anyone seeking a deeper comprehension of the molecular world. This detailed exploration provides a strong foundation for understanding more advanced concepts in chemistry and the intricacies of molecular behavior.
Latest Posts
Latest Posts
-
Difference Between Molar Mass And Atomic Mass
Apr 02, 2025
-
Write In Standard Form The Equation Of Each Line
Apr 02, 2025
-
What Does Fad Stand For In Biology
Apr 02, 2025
-
The Outermost Layer Of The Heart Is Called The
Apr 02, 2025
-
Which Of The Following Are Channels Of Nonverbal Communication
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Does A Sigma Bond Look Like . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
