Do Ionic Bonds Dissolve In Water
Muz Play
Mar 22, 2025 · 6 min read
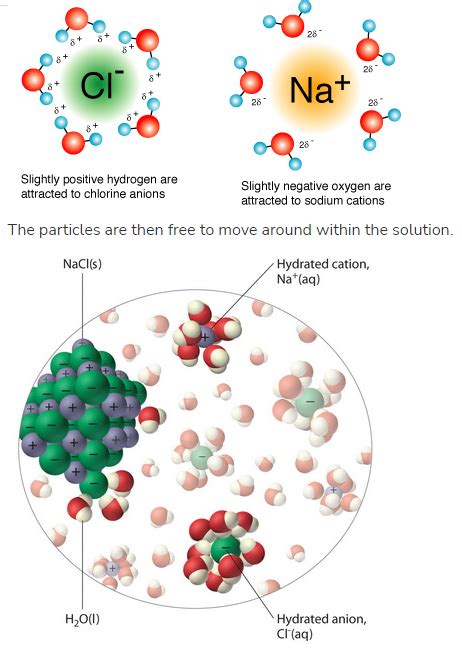
Table of Contents
Do Ionic Bonds Dissolve in Water? A Deep Dive into Polarity, Hydration, and Solubility
Ionic bonds, the electrostatic forces of attraction between oppositely charged ions, are fundamental to the structure of many compounds. Understanding their behavior in water, a ubiquitous solvent, is crucial in various fields, from chemistry and biology to environmental science and materials engineering. This comprehensive article explores the intricate dance between ionic compounds and water molecules, revealing why some ionic bonds dissolve readily while others exhibit limited solubility.
The Polar Nature of Water: The Key to Dissolution
Water's unique properties stem largely from its polarity. The oxygen atom, being more electronegative than the hydrogen atoms, attracts the shared electrons more strongly. This creates a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge results in a dipolar molecule, resembling a tiny magnet with a positive and negative end. This polarity is the driving force behind the dissolution of many ionic compounds.
Hydrogen Bonding: Strengthening Water's Polarity
The polarity of water is further enhanced by hydrogen bonding. The slightly positive hydrogen atoms of one water molecule are attracted to the slightly negative oxygen atoms of neighboring water molecules. This creates a strong network of intermolecular forces, influencing water's high boiling point, surface tension, and its ability to dissolve ionic compounds.
The Dissolution Process: A Step-by-Step Explanation
The dissolution of an ionic compound in water is a complex process involving several key steps:
1. Ion-Dipole Interactions: The Initial Attraction
When an ionic compound, such as sodium chloride (NaCl), is added to water, the polar water molecules approach the crystal lattice. The partially negative oxygen atoms of water are attracted to the positively charged sodium ions (Na+), while the partially positive hydrogen atoms are attracted to the negatively charged chloride ions (Cl-). These attractions are known as ion-dipole interactions.
2. Overcoming Lattice Energy: Breaking the Bonds
The strength of the ionic bond within the crystal lattice, quantified by its lattice energy, determines how easily the compound dissolves. The ion-dipole interactions between water molecules and the ions must overcome this lattice energy to pull the ions apart. This is an energy-intensive process, requiring sufficient kinetic energy from the water molecules. Higher temperatures generally increase the kinetic energy, facilitating dissolution.
3. Hydration: Surrounding the Ions
Once the ions are separated from the crystal lattice, they become surrounded by a shell of water molecules. This process is called hydration. The water molecules orient themselves so that their partially charged ends interact with the oppositely charged ions. The hydrated ions are now stabilized, preventing them from recombining to form the solid crystal. The strength of hydration depends on the size and charge of the ions. Smaller, highly charged ions generally experience stronger hydration.
4. Entropy and Dissolution: The Role of Disorder
The dissolution process is also influenced by entropy, the measure of disorder in a system. A solid crystal represents a highly ordered state, while a solution of dissolved ions exhibits greater disorder. The increase in entropy during dissolution favors the process, contributing to the overall solubility of the ionic compound.
Factors Affecting the Solubility of Ionic Compounds
While the general principle is that ionic compounds dissolve in water due to ion-dipole interactions, several factors modify this principle:
1. Lattice Energy: The Strength of the Ionic Bond
Compounds with high lattice energy, indicating strong ionic bonds, tend to be less soluble. The energy required to break these strong bonds may exceed the energy gained from ion-dipole interactions and hydration. For example, many metal oxides and sulfides exhibit low solubility due to their high lattice energies.
2. Ion Size and Charge: Hydration's Influence
Smaller ions with higher charges have stronger interactions with water molecules. This stronger hydration energy contributes to greater solubility. Conversely, larger ions with lower charges have weaker hydration and lower solubility.
3. Polarity of the Solvent: Beyond Water
The dissolution of ionic compounds is not limited to water. Other polar solvents, such as ethanol and methanol, can also dissolve ionic compounds, albeit often to a lesser extent than water. The polarity of the solvent, as reflected in its dielectric constant, determines its ability to weaken the electrostatic interactions within the ionic crystal and stabilize the separated ions.
4. Temperature: Kinetic Energy's Role
Increasing temperature increases the kinetic energy of water molecules, aiding in overcoming lattice energy and enhancing dissolution. However, the effect of temperature on solubility varies depending on the specific ionic compound. For most ionic compounds, solubility increases with temperature.
5. Common Ion Effect: Reducing Solubility
The presence of a common ion in the solution can decrease the solubility of an ionic compound. This effect is based on Le Chatelier's principle, which states that a system at equilibrium will shift to counteract any stress applied to it. Adding a common ion increases the concentration of one of the ions in the solution, causing the equilibrium to shift towards the formation of the solid ionic compound, thus reducing its solubility.
Exceptions and Complications: Not All Ionic Bonds Dissolve Easily
While many ionic compounds dissolve readily in water, some exceptions exist:
1. Low Solubility Ionic Compounds: The Balance of Forces
Some ionic compounds have limited solubility even though ion-dipole interactions are favorable. This happens when the lattice energy is high enough to counteract the hydration energy. These compounds may exhibit minimal dissolution, forming a saturated solution with a small concentration of dissolved ions.
2. Insoluble Ionic Compounds: Overwhelming Lattice Energy
Some ionic compounds are virtually insoluble in water. The lattice energy far surpasses the energy gained from hydration and ion-dipole interactions. These compounds remain essentially undissolved even when submerged in water. Examples include many metal sulfides and carbonates.
3. Complex Ion Formation: Influencing Solubility
The presence of certain ligands or complexing agents can significantly affect the solubility of ionic compounds. These ligands can form coordinate covalent bonds with metal ions, creating complex ions. The formation of complex ions can alter the overall charge and size of the metal ion, subsequently impacting its interaction with water molecules and altering its solubility.
Applications and Significance
Understanding the solubility of ionic compounds in water has widespread applications:
- Medicine: Solubility is a critical factor in drug delivery. Drugs must be soluble enough to be absorbed by the body.
- Environmental Science: The solubility of various pollutants influences their transport and fate in the environment.
- Agriculture: The solubility of fertilizers determines their availability to plants.
- Materials Science: Solubility is crucial in designing new materials and understanding their properties.
- Chemical Engineering: Many industrial processes rely on the dissolution and precipitation of ionic compounds.
Conclusion: A Dynamic Equilibrium
The dissolution of ionic compounds in water is a dynamic equilibrium between the forces of attraction within the crystal lattice and the forces of attraction between ions and water molecules. While the polarity of water and the ion-dipole interactions generally favor dissolution, factors like lattice energy, ion size and charge, temperature, and the presence of common ions all play significant roles in determining the extent of solubility. Understanding these factors is fundamental to comprehending the behavior of ionic compounds in aqueous solutions and their wide-ranging applications across diverse scientific and technological fields. Further research continues to refine our understanding of this intricate process, constantly revealing new nuances and influencing technological advancements.
Latest Posts
Latest Posts
-
Diffusion Across A Biological Membrane Is Called
Mar 22, 2025
-
C Double Bond C Ir Spectrum
Mar 22, 2025
-
Dot Product Of A Vector With Itself
Mar 22, 2025
-
Examples Of Type I And Type Ii Errors
Mar 22, 2025
-
The Composition Of Heterogeneous Mixtures Is
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Bonds Dissolve In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
