Do Ionic Bonds Have High Melting Points
Muz Play
Mar 21, 2025 · 5 min read
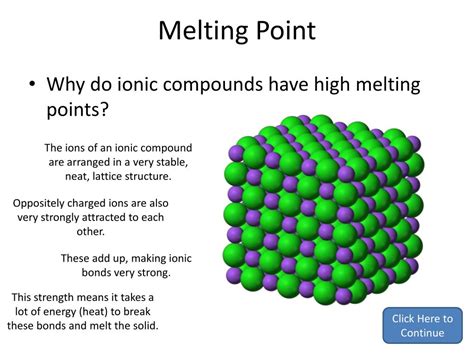
Table of Contents
Do Ionic Bonds Have High Melting Points? A Deep Dive into Ionic Compounds
Ionic bonds, characterized by the strong electrostatic attraction between oppositely charged ions, are renowned for conferring high melting points on the compounds they form. But why? Understanding this requires a delve into the fundamental nature of ionic bonding and the forces that govern the solid state. This article will explore this phenomenon in detail, examining the factors influencing melting points, exceptions to the rule, and real-world applications.
The Essence of Ionic Bonding and Crystal Structure
Ionic bonds arise from the electrostatic attraction between a cation (positively charged ion) and an anion (negatively charged ion). This transfer of electrons, typically from a metal to a non-metal, results in a stable arrangement where each ion achieves a noble gas electron configuration. This strong electrostatic force is the key to understanding the high melting points.
Lattice Energy: The Glue Holding Ions Together
The strength of the ionic bond is quantified by lattice energy. Lattice energy is defined as the energy required to completely separate one mole of a solid ionic compound into its constituent gaseous ions. A higher lattice energy indicates a stronger ionic bond. The higher the lattice energy, the higher the melting point.
The magnitude of lattice energy depends on several factors:
-
Charge of the Ions: Higher charges on the ions lead to stronger electrostatic attraction and thus higher lattice energy. For example, the lattice energy of MgO (Mg²⁺ and O²⁻) is significantly higher than that of NaCl (Na⁺ and Cl⁻).
-
Size of the Ions: Smaller ions result in a shorter distance between the cation and anion, leading to a stronger electrostatic attraction and higher lattice energy. Smaller ions are more easily polarizable (ability to distort their electron cloud). This enhances attractive forces and contributes to a higher melting point.
-
Crystal Structure: The arrangement of ions in the crystal lattice also influences lattice energy. A more efficient packing arrangement leads to stronger interactions and a higher melting point.
High Melting Points: A Consequence of Strong Interactions
The high melting points observed in ionic compounds are a direct consequence of the strong electrostatic forces between the ions within the crystal lattice. To melt an ionic compound, sufficient energy must be supplied to overcome these strong attractive forces and break down the ordered arrangement of ions. This energy requirement translates to high melting temperatures.
Overcoming Electrostatic Attraction: The Energy Barrier
Imagine the ions in an ionic crystal as being held together by countless springs. Melting requires stretching and breaking these "springs," which demands a significant input of energy. This energy input is primarily in the form of heat. The stronger the electrostatic attraction (i.e., the higher the lattice energy), the more energy is required to overcome these forces, and consequently, the higher the melting point.
Factors Affecting Melting Points: Beyond the Basics
While the fundamental principle is straightforward, several factors can subtly influence the melting points of ionic compounds:
-
Covalent Character: Some ionic compounds exhibit a degree of covalent character, particularly when there is a significant difference in electronegativity between the cation and anion. This covalent character can slightly weaken the overall bonding and lower the melting point.
-
Polarizability: As mentioned earlier, the polarizability of ions influences the strength of attraction. Larger, more polarizable anions can lead to stronger interactions and higher melting points, deviating slightly from the simple size-charge rule.
-
Presence of Water: Hydration of ions can significantly affect the melting point. Water molecules can interact with ions, weakening the electrostatic forces and lowering the melting point. Anhydrous salts usually have significantly higher melting points than their hydrated counterparts.
-
Impurities: The presence of impurities in an ionic crystal can disrupt the regular lattice structure and weaken the ionic bonds, leading to a lower melting point.
Exceptions to the Rule: When Ionic Compounds Melt at Lower Temperatures
While many ionic compounds exhibit high melting points, there are exceptions. These exceptions often arise from factors that weaken the overall electrostatic interactions within the crystal lattice:
-
Large Ion Size: When ions are very large, the electrostatic attraction weakens due to the increased distance between the cation and anion. This results in lower melting points.
-
Low Charge Density: Ions with low charge density have weaker electrostatic interactions, leading to lower melting points compared to those with high charge density.
-
Complex Ion Formation: The formation of complex ions can disrupt the regular crystal lattice and reduce the strength of the ionic interactions, leading to lower melting points.
Real-World Applications: High Melting Points in Action
The high melting points of many ionic compounds are exploited in various real-world applications:
-
High-Temperature Materials: Ionic compounds like ceramics and certain oxides are used in high-temperature applications due to their thermal stability. They are employed in furnace linings, heat shields, and high-temperature industrial processes.
-
Electrolytes: Ionic compounds are crucial components in electrolytes, the solutions that conduct electricity in batteries and fuel cells. Their ability to dissociate into ions facilitates the flow of charge.
-
Metallurgy: Some ionic compounds act as fluxes in metallurgical processes, assisting in the removal of impurities from molten metals.
-
Mineral Composition: The high melting points of minerals in the Earth's crust contribute to the stability of geological formations. The melting and solidification of these ionic compounds influence volcanic activity and rock formation.
Conclusion: A Strong Bond, a High Melting Point
The high melting points of ionic compounds are a direct consequence of the strong electrostatic attractions between the oppositely charged ions in their crystal lattices. This strong bonding, quantified by lattice energy, requires a substantial amount of energy to overcome, resulting in high melting temperatures. While exceptions exist, influenced by factors such as ion size, charge, and the presence of covalent character, the correlation between ionic bonding and high melting points remains a fundamental principle in chemistry with significant implications across various scientific and technological domains. Understanding the nuances of ionic bonding and lattice energy is key to predicting and manipulating the properties of these vital materials.
Latest Posts
Latest Posts
-
Partial Fraction Decomposition With Long Division
Mar 22, 2025
-
What Is A Lone Pair Of Electrons
Mar 22, 2025
-
The Process Of Lysing A Cell Results In
Mar 22, 2025
-
Label The Spinal Nerves And Their Plexuses
Mar 22, 2025
-
Are Daughter Cells Identical To Each Other In Meiosis
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Bonds Have High Melting Points . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
