Do Ionic Compounds Become Electrolytes In Water
Muz Play
Mar 16, 2025 · 6 min read
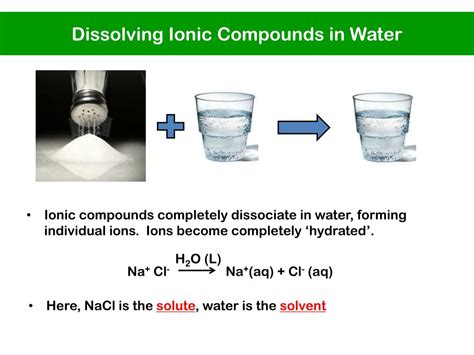
Table of Contents
Do Ionic Compounds Become Electrolytes in Water? A Deep Dive into Dissolution and Conductivity
Ionic compounds, characterized by the electrostatic attraction between positively and negatively charged ions, exhibit unique behavior when introduced to water. Understanding whether and how they become electrolytes is crucial in various fields, from chemistry and biology to engineering and medicine. This comprehensive exploration delves into the process of ionic compound dissolution, the formation of electrolytes, and the factors influencing conductivity.
The Nature of Ionic Compounds and Water
Before examining the interaction, let's establish a foundation. Ionic compounds, like sodium chloride (NaCl) or table salt, are formed through the transfer of electrons from a metal atom (like sodium) to a non-metal atom (like chlorine). This transfer creates ions: positively charged cations (Na⁺) and negatively charged anions (Cl⁻), held together by strong electrostatic forces in a crystal lattice structure.
Water (H₂O), on the other hand, is a polar molecule. This means it possesses a slightly positive end (near the hydrogen atoms) and a slightly negative end (near the oxygen atom). This polarity is key to understanding its interaction with ionic compounds.
The Dissolution Process: Breaking Down the Crystal Lattice
When an ionic compound is added to water, the polar water molecules begin to interact with the ions at the surface of the crystal lattice. This interaction is driven by the attractive forces between the partially positive hydrogen atoms of water and the negatively charged anions, and the attractive forces between the partially negative oxygen atoms of water and the positively charged cations.
This process is often referred to as hydration. The water molecules effectively surround the individual ions, creating a hydration shell. This shell shields the ions from each other, weakening the electrostatic forces holding the crystal lattice together.
The Role of Hydration Energy
The energy released during the hydration process is called hydration energy. This energy is crucial in determining whether an ionic compound will dissolve in water. If the hydration energy is sufficient to overcome the lattice energy (the energy holding the ions together in the crystal), the compound will dissolve. The higher the hydration energy relative to the lattice energy, the more soluble the ionic compound will be.
Factors Affecting Solubility
Several factors influence the solubility of ionic compounds in water:
-
Charge Density: Ions with higher charge density (higher charge and smaller size) have stronger interactions with water molecules, leading to higher hydration energy and greater solubility. For example, MgCl₂ is more soluble than NaCl because Mg²⁺ has a higher charge density than Na⁺.
-
Lattice Energy: Compounds with high lattice energy require more energy to break apart, making them less soluble. This is influenced by the size and charge of the ions – smaller ions and higher charges lead to stronger attractions and higher lattice energy.
-
Temperature: Increasing temperature generally increases the solubility of most ionic compounds. The increased kinetic energy helps overcome the lattice energy more effectively.
-
Common Ion Effect: The presence of a common ion in solution decreases the solubility of the ionic compound. For example, adding NaCl to a saturated solution of AgCl will decrease the solubility of AgCl because of the common ion Cl⁻.
Formation of Electrolytes: Conductivity in Solution
Once an ionic compound dissolves in water, it dissociates into its constituent ions. These freely moving ions are what make the solution an electrolyte – a substance that conducts electricity.
Mechanism of Electrical Conductivity
The electrical conductivity arises from the ability of the ions to carry an electric current. When an electric field is applied across the solution, the positively charged cations move towards the negative electrode (cathode), and the negatively charged anions move towards the positive electrode (anode). This movement of charged particles constitutes the electric current.
Strong vs. Weak Electrolytes
The extent to which an ionic compound dissociates into ions determines whether it's a strong or weak electrolyte.
-
Strong Electrolytes: These compounds dissociate completely or almost completely in water, producing a high concentration of ions and exhibiting high electrical conductivity. Examples include NaCl, KCl, and most other alkali metal halides.
-
Weak Electrolytes: These compounds only partially dissociate in water, producing a relatively low concentration of ions and exhibiting low electrical conductivity. Examples include weak acids and weak bases like acetic acid (CH₃COOH) and ammonia (NH₃). The equilibrium between the undissociated molecules and the ions determines the degree of dissociation.
Factors Affecting Electrolyte Strength
Besides the intrinsic properties of the ionic compound, other factors can influence the electrolyte strength of a solution:
-
Concentration: Increasing the concentration of a strong electrolyte increases the conductivity proportionally. However, with weak electrolytes, the increase in conductivity is less pronounced due to the limited dissociation.
-
Temperature: Similar to solubility, increased temperature generally enhances the conductivity of electrolyte solutions by increasing the mobility of ions.
-
Solvent: Water is an excellent solvent for many ionic compounds, but other polar solvents can also dissolve ionic compounds and create electrolyte solutions. The dielectric constant of the solvent plays a significant role in its ability to dissolve ionic compounds. Higher dielectric constants allow for better separation of ions.
-
Presence of Other Ions: The presence of other ions in the solution can influence conductivity through ion-ion interactions. These interactions can either increase or decrease conductivity depending on the specific ions involved.
Applications of Ionic Compounds and Electrolytes
The properties of ionic compounds and their ability to form electrolytes have widespread applications:
-
Biological Systems: Electrolytes like sodium, potassium, calcium, and chloride ions are essential for various biological processes, including nerve impulse transmission, muscle contraction, and maintaining osmotic balance.
-
Batteries: Many batteries rely on the movement of ions in an electrolyte solution to generate electric current. The electrolyte facilitates the flow of charge between the electrodes.
-
Electroplating: Electroplating uses electrolyte solutions to deposit a thin layer of metal onto a surface. The process involves the reduction of metal ions at the cathode.
-
Water Treatment: Electrolytes play a role in water treatment processes, such as coagulation and disinfection.
-
Medicine: Electrolyte solutions are used intravenously to treat dehydration and electrolyte imbalances.
Conclusion: A Dynamic Interaction
The behavior of ionic compounds in water is a complex interplay between lattice energy, hydration energy, and the inherent properties of both the compound and the solvent. The resulting electrolyte solutions are crucial for a multitude of applications, highlighting the significance of understanding the dissolution process and the factors influencing conductivity. This detailed examination underscores the importance of considering various parameters to accurately predict and utilize the properties of these important substances. Further research continues to refine our understanding of these interactions, leading to advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Compounds Become Electrolytes In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
