Do Ionic Compounds Have A High Melting Point
Muz Play
Mar 15, 2025 · 6 min read
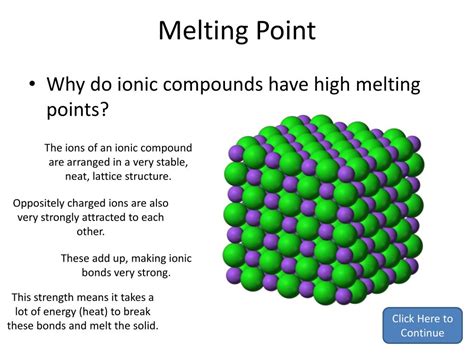
Table of Contents
Do Ionic Compounds Have a High Melting Point? A Deep Dive into Ionic Bonding and its Properties
Ionic compounds are renowned for their high melting points, a characteristic stemming directly from the strong electrostatic forces holding their constituent ions together. Understanding why this is the case requires a deeper exploration of the nature of ionic bonding, the factors influencing melting point, and the exceptions that can arise. This comprehensive article will delve into these aspects, providing a robust understanding of the relationship between ionic bonding and melting point.
Understanding Ionic Bonding: The Foundation of High Melting Points
Ionic compounds form when atoms with significantly different electronegativities interact. Electronegativity is the measure of an atom's ability to attract electrons in a chemical bond. When a highly electronegative atom (like a halogen, such as chlorine) encounters a metal with low electronegativity (like sodium), the electronegative atom effectively steals an electron from the metal atom. This transfer of electrons results in the formation of ions: positively charged cations (metal ions) and negatively charged anions (non-metal ions).
The electrostatic attraction between these oppositely charged ions is incredibly strong. This strong attraction is known as the electrostatic force, and it's the driving force behind the formation of the ionic bond and the resultant crystal lattice structure. These electrostatic forces are significantly stronger than the intermolecular forces found in covalent and metallic compounds, which directly contributes to the high melting points observed in ionic compounds.
Coulomb's Law: Quantifying the Electrostatic Attraction
The strength of the electrostatic attraction between ions is governed by Coulomb's Law:
F = k * (q1 * q2) / r²
Where:
- F represents the force of attraction
- k is Coulomb's constant
- q1 and q2 are the magnitudes of the charges of the ions
- r is the distance between the centers of the ions
This equation reveals two key factors influencing the melting point:
-
Charge Magnitude (q1 and q2): Higher charges on the ions lead to stronger electrostatic attraction and thus a higher melting point. For example, a compound with +2 and -2 ions will have a significantly higher melting point than one with +1 and -1 ions.
-
Ionic Radius (r): Smaller ionic radii result in a shorter distance between the ions, leading to a stronger electrostatic force and a higher melting point. As the distance (r) increases, the force of attraction (F) decreases proportionally to the square of the distance.
The Crystalline Structure: A Giant Network of Ionic Bonds
Ionic compounds don't exist as individual molecules; instead, they form a three-dimensional crystal lattice. This lattice is a highly ordered arrangement of cations and anions, with each ion surrounded by ions of opposite charge. This arrangement maximizes the electrostatic attractions between ions, contributing to the overall stability and high melting point of the compound.
To melt an ionic compound, you need to overcome these strong electrostatic attractions throughout the entire crystal lattice. This requires a substantial amount of energy, hence the high melting point. The energy required is directly proportional to the strength of the ionic bonds.
Factors Affecting Crystal Lattice Energy
The lattice energy is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. It's a direct measure of the strength of the ionic bonds and is highly correlated with the melting point. Several factors influence lattice energy:
-
Charge Density: Ions with high charge density (high charge and small size) lead to higher lattice energies and higher melting points.
-
Ionic Size: Smaller ions result in stronger electrostatic attractions and higher lattice energies.
-
Arrangement of Ions: The specific arrangement of ions within the crystal lattice can also slightly affect the overall lattice energy.
Exceptions and Nuances: When Ionic Compounds Have Lower Melting Points
While generally true, there are exceptions to the rule that ionic compounds possess high melting points. Several factors can lead to deviations from this general trend:
-
Covalent Character: Some ionic compounds exhibit partial covalent character, especially when the electronegativity difference between the cation and anion is not extremely large. This covalent character weakens the overall electrostatic attraction, resulting in a lower melting point.
-
Polarizability: Larger ions are more easily polarized, meaning their electron clouds can be distorted. This distortion reduces the effectiveness of the electrostatic attraction and can lead to lower melting points.
-
Structural Complexity: The complexity of the crystal structure can also influence the melting point. A more complex structure might have weaker average bond strengths compared to a simpler structure.
-
Hydrogen Bonding: In some cases, hydrogen bonding can influence the melting point. The presence of strong hydrogen bonds can increase the energy needed to melt the compound, partially compensating for weaker ionic bonds.
Comparing Ionic Melting Points with Other Compound Types
To fully appreciate the high melting points of ionic compounds, it's helpful to compare them with other types of compounds:
-
Covalent Compounds: Covalent compounds are characterized by shared electrons between atoms. These bonds are generally weaker than ionic bonds, resulting in much lower melting points. The intermolecular forces in covalent compounds, such as van der Waals forces, dipole-dipole interactions, and hydrogen bonds are weaker than the electrostatic forces in ionic compounds.
-
Metallic Compounds: Metallic compounds have a sea of delocalized electrons that hold the metal atoms together. The strength of metallic bonds varies considerably depending on the metal, resulting in a wide range of melting points. However, many metals have melting points significantly lower than those of ionic compounds with similar molar masses.
Practical Applications and Significance
The high melting points of ionic compounds have significant practical implications. Many high-temperature applications rely on the thermal stability of ionic materials. Examples include:
-
High-temperature ceramics: Ionic compounds form the basis of many high-temperature ceramics used in industrial applications and high-temperature furnaces.
-
Electrolytes: Ionic compounds are essential in batteries and fuel cells as electrolytes, facilitating the movement of ions and generating electrical current.
-
Refractory materials: Many ionic compounds exhibit exceptional refractory properties, meaning they resist high temperatures and erosion, making them crucial in various industrial processes.
-
Mineral compositions: Understanding the high melting points of ionic compounds is crucial in geology and materials science for interpreting the composition and behavior of minerals and rocks within the Earth's crust.
Conclusion: A Strong Bond Leads to High Temperature Stability
In summary, the high melting points of ionic compounds are a direct consequence of the strong electrostatic forces between oppositely charged ions within their crystalline lattice structure. While exceptions exist due to factors like covalent character, polarizability, and structural complexity, the fundamental principle remains: the stronger the ionic bonds, the higher the melting point. This understanding is crucial across diverse scientific and engineering disciplines, highlighting the importance of ionic compounds in numerous technological applications and natural phenomena. Further research continues to refine our understanding of these interactions and explore new applications of these fascinating materials.
Latest Posts
Latest Posts
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Compounds Have A High Melting Point . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
