Do Polar Covalent Bonds Share Electrons Equally
Muz Play
Apr 01, 2025 · 6 min read
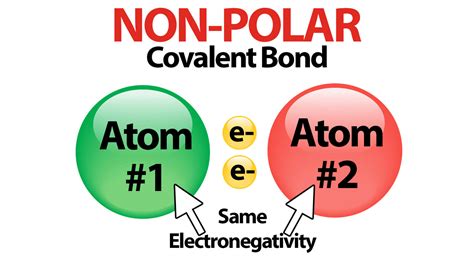
Table of Contents
Do Polar Covalent Bonds Share Electrons Equally? A Deep Dive into Chemical Bonding
Understanding chemical bonding is fundamental to grasping the behavior of matter. One crucial aspect of this understanding involves differentiating between various types of bonds, specifically focusing on the nuances of covalent bonding and its polar variant. This article delves into the question: Do polar covalent bonds share electrons equally? The short answer is no, but the intricacies behind this inequality are far more fascinating than a simple "yes" or "no."
The Fundamentals of Covalent Bonding
Before tackling the polarity aspect, let's establish a firm grasp on the basics of covalent bonding. Covalent bonds are formed when two atoms share one or more pairs of electrons. This sharing occurs because both atoms involved benefit energetically from acquiring a more stable electron configuration, often resembling that of a noble gas. This shared electron pair resides in a region of space between the two atomic nuclei, effectively acting as a "glue" that holds the atoms together.
Examples of Covalent Bonds
Simple examples abound. Consider the hydrogen molecule (H₂). Each hydrogen atom has one electron. By sharing their single electrons, both atoms achieve a stable electron configuration of two electrons, effectively mimicking the noble gas helium. Similarly, the oxygen molecule (O₂) involves the sharing of two pairs of electrons (a double bond) to achieve a stable octet.
The Concept of Electronegativity: The Key to Polarity
The crucial factor differentiating between nonpolar and polar covalent bonds is electronegativity. Electronegativity is a measure of an atom's ability to attract shared electrons in a chemical bond. Atoms with high electronegativity exert a stronger pull on the shared electrons than atoms with low electronegativity.
The periodic table provides a valuable tool for understanding electronegativity trends. Electronegativity generally increases across a period (from left to right) and decreases down a group (from top to bottom). Fluorine (F) is the most electronegative element, while Francium (Fr) is among the least.
Nonpolar Covalent Bonds: The Equal Sharing
In nonpolar covalent bonds, the atoms involved have similar or identical electronegativities. Consequently, the shared electrons are attracted equally by both atoms. The electron distribution remains symmetrical, with no significant build-up of charge at either end of the bond.
Examples of Nonpolar Covalent Bonds
The hydrogen molecule (H₂) is a prime example of a nonpolar covalent bond. Since both hydrogen atoms have identical electronegativities, the shared electron pair is equally distributed between them. Similarly, bonds between identical atoms, such as in chlorine gas (Cl₂), are also nonpolar.
Polar Covalent Bonds: Unequal Sharing - The Heart of the Matter
Now, we arrive at the central theme: polar covalent bonds. In polar covalent bonds, the atoms involved have significantly different electronegativities. This difference results in an unequal sharing of electrons. The atom with the higher electronegativity attracts the shared electron pair more strongly, leading to a greater electron density around that atom.
This unequal distribution of electrons generates a dipole moment, a measure of the separation of positive and negative charges within the molecule. The more electronegative atom acquires a partial negative charge (δ-), while the less electronegative atom acquires a partial positive charge (δ+).
Visualizing the Unequal Sharing
Imagine a tug-of-war between two individuals of unequal strength. The stronger individual (the more electronegative atom) pulls the rope (the shared electrons) closer to themselves, resulting in an uneven distribution. This analogy aptly describes the unequal sharing of electrons in polar covalent bonds.
Examples of Polar Covalent Bonds
Water (H₂O) serves as a quintessential example. Oxygen is significantly more electronegative than hydrogen. Consequently, the shared electrons are pulled more strongly towards the oxygen atom, resulting in a partial negative charge on oxygen (δ-) and partial positive charges on the hydrogen atoms (δ+). This polarity is responsible for many of water's unique properties, such as its high boiling point and its ability to act as a solvent. Other common examples include hydrogen fluoride (HF) and ammonia (NH₃).
The Electronegativity Difference and Bond Type
The magnitude of the electronegativity difference between two atoms is a key determinant of the type of bond formed.
- Nonpolar Covalent: Electronegativity difference close to zero (generally less than 0.5).
- Polar Covalent: Electronegativity difference between 0.5 and 1.7.
- Ionic: Electronegativity difference greater than 1.7.
It's important to remember that these values are guidelines, and the actual bond character can fall on a spectrum. For example, a bond with an electronegativity difference of 1.5 might exhibit characteristics of both polar covalent and ionic bonding.
Consequences of Polarity: Molecular Dipole Moments and Intermolecular Forces
The polarity of a molecule significantly influences its properties and behavior. The presence of a molecular dipole moment affects:
-
Melting and boiling points: Polar molecules generally have higher melting and boiling points than nonpolar molecules of similar size due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding).
-
Solubility: Polar molecules tend to dissolve better in polar solvents (like water) due to the ability to form favorable interactions. Nonpolar molecules dissolve better in nonpolar solvents.
-
Reactivity: The partial charges in polar molecules can influence their reactivity, making them more susceptible to certain types of chemical reactions.
Advanced Concepts: Resonance and Delocalization
In certain molecules, the electron distribution is not easily represented by a single Lewis structure. Resonance structures are used to depict these molecules, indicating that the electrons are delocalized over multiple atoms. While this delocalization can complicate the determination of partial charges, the fundamental principle of unequal electron sharing in polar covalent bonds remains valid.
Beyond the Basics: Percentage Ionic Character
The degree of polarity in a covalent bond can also be quantified by the percentage ionic character. This is a measure of how much the bond resembles an ionic bond, and it’s related to the electronegativity difference between the bonded atoms. While the electronegativity difference gives a qualitative indication of polarity, the percentage ionic character provides a quantitative measure. However, it's crucial to understand that even bonds with a high percentage ionic character still involve a degree of electron sharing, albeit highly unequal.
Conclusion: A Spectrum of Bonding
In conclusion, polar covalent bonds do not share electrons equally. The unequal sharing stems from the difference in electronegativity between the atoms involved. The greater the electronegativity difference, the more polar the bond becomes. Understanding this concept is essential for predicting and interpreting the properties and behavior of countless molecules in chemistry and beyond. The entire picture of chemical bonding is a fascinating spectrum, ranging from purely nonpolar to predominantly ionic, with polar covalent bonds occupying the significant middle ground. The exploration of this spectrum continues to drive cutting-edge research in material science, drug discovery, and many other scientific fields.
Latest Posts
Latest Posts
-
What Organelles Do Plants Have That Animals Dont
Apr 02, 2025
-
Select The Five Major Mechanisms Of Antimicrobial Resistance
Apr 02, 2025
-
What Are The Three Characteristics Of All Metals
Apr 02, 2025
-
Using The General Properties Of Reaction Enthalpy
Apr 02, 2025
-
Graphing Sine And Cosine Worksheet Answers
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Do Polar Covalent Bonds Share Electrons Equally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
