Do Weak Bases Have Strong Conjugate Acids
Muz Play
Mar 26, 2025 · 6 min read
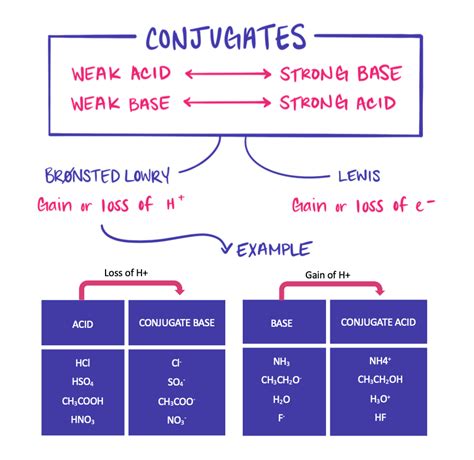
Table of Contents
Do Weak Bases Have Strong Conjugate Acids? Understanding Acid-Base Conjugate Pairs
The relationship between a weak base and its conjugate acid is a cornerstone of acid-base chemistry. Understanding this relationship is crucial for predicting the behavior of solutions and for mastering many concepts in chemistry, including buffer solutions, titrations, and equilibrium calculations. The simple answer to the question, "Do weak bases have strong conjugate acids?", is no. However, the nuances of this relationship require a deeper exploration. This article will delve into the intricacies of weak bases, strong conjugate acids, and the equilibrium that governs their behavior.
Defining Weak Bases and Conjugate Acids
Before exploring the relationship, let's define our key terms:
Weak Bases:
A weak base is a substance that partially ionizes in water, meaning it doesn't completely dissociate into its ions. Instead, it establishes an equilibrium between the un-ionized base and its ions. This partial ionization results in a solution with a relatively low concentration of hydroxide ions (OH⁻) compared to a strong base. Examples include ammonia (NH₃), methylamine (CH₃NH₂), and many organic amines.
The general reaction of a weak base (B) with water is:
B(aq) + H₂O(l) ⇌ BH⁺(aq) + OH⁻(aq)
Where:
- B represents the weak base
- BH⁺ represents its conjugate acid
- OH⁻ represents hydroxide ions
The equilibrium constant for this reaction is the base dissociation constant, K<sub>b</sub>. A smaller K<sub>b</sub> value indicates a weaker base.
Conjugate Acids:
A conjugate acid is the species formed when a base accepts a proton (H⁺). It's the species that results from the addition of a proton to the base. In the example above, BH⁺ is the conjugate acid of the weak base B. Essentially, it's the acidic form of the base.
The Relationship Between Weak Bases and Their Conjugate Acids: An Equilibrium Perspective
The strength of a conjugate acid is inversely related to the strength of its conjugate base. This means:
-
A weak base has a relatively strong conjugate acid. While not "strong" in the absolute sense (like HCl), the conjugate acid will be significantly more likely to donate a proton compared to the original weak base's ability to accept one.
-
A strong base has a very weak conjugate acid. The conjugate acid of a strong base is so weak that it essentially doesn't donate protons in aqueous solution.
This inverse relationship stems from the equilibrium constant expressions. Consider the K<sub>b</sub> for a weak base and the K<sub>a</sub> for its conjugate acid:
K<sub>w</sub> = K<sub>a</sub> * K<sub>b</sub>
Where K<sub>w</sub> is the ion product of water (1.0 x 10⁻¹⁴ at 25°C). This equation shows the inherent link between the acidity of the conjugate acid and the basicity of the original base. If K<sub>b</sub> is small (weak base), then K<sub>a</sub> must be relatively large to satisfy this relationship. This larger K<sub>a</sub> signifies a stronger conjugate acid.
Why "Strong" Conjugate Acid is Relative
It's crucial to understand that the term "strong" in this context is relative. While the conjugate acid of a weak base is stronger than the base itself, it's not necessarily a strong acid in the absolute sense like HCl, H₂SO₄, or HNO₃. These strong acids completely dissociate in water. The conjugate acids of weak bases, while stronger than their conjugate bases, still only partially dissociate, establishing an equilibrium in solution.
Examples Illustrating the Relationship
Let's consider some examples to solidify our understanding:
1. Ammonia (NH₃):
Ammonia is a weak base. Its reaction with water is:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
The conjugate acid of ammonia is the ammonium ion (NH₄⁺). While NH₄⁺ is a weak acid, it's considerably stronger than NH₃. It can donate a proton, albeit not completely.
2. Methylamine (CH₃NH₂):
Methylamine is another weak base, similar to ammonia. Its reaction with water is:
CH₃NH₂(aq) + H₂O(l) ⇌ CH₃NH₃⁺(aq) + OH⁻(aq)
The conjugate acid, methylammonium ion (CH₃NH₃⁺), is a weak acid, yet stronger than methylamine.
3. Acetate Ion (CH₃COO⁻):
The acetate ion is a weak base. Its reaction with water is:
CH₃COO⁻(aq) + H₂O(l) ⇌ CH₃COOH(aq) + OH⁻(aq)
The conjugate acid is acetic acid (CH₃COOH), a weak acid but stronger than the acetate ion.
Practical Implications and Applications
Understanding the relationship between weak bases and their conjugate acids is crucial in various chemical applications:
1. Buffer Solutions:
Buffer solutions resist changes in pH when small amounts of acid or base are added. They are typically composed of a weak acid and its conjugate base (or a weak base and its conjugate acid). The conjugate acid-base pair works together to neutralize added H⁺ or OH⁻ ions, minimizing pH changes.
2. Titrations:
Titrations involve the gradual addition of a solution of known concentration (titrant) to a solution of unknown concentration. Understanding the conjugate acid-base relationships is critical in determining the equivalence point and calculating the unknown concentration.
3. Pharmaceutical Applications:
Many drugs and pharmaceuticals act as weak acids or bases. Understanding their conjugate acid-base behavior is crucial for predicting their solubility, absorption, and efficacy in the body. pH adjustments are often used to control the ionization state of drugs and improve their bioavailability.
4. Environmental Chemistry:
Many environmental processes involve acid-base reactions. Understanding the conjugate acid-base relationships of pollutants and natural substances is important for assessing their environmental impact and developing remediation strategies.
Factors Influencing Conjugate Acid Strength
While the strength of a conjugate acid is inversely related to the strength of its conjugate base, other factors can also influence its acidity:
-
Inductive Effects: Electron-withdrawing groups can increase the acidity of a conjugate acid by stabilizing the negative charge on the conjugate base.
-
Resonance Effects: Resonance stabilization of the conjugate base can also increase the acidity of the conjugate acid.
-
Size and Electronegativity: The size and electronegativity of the atoms in the conjugate acid can influence its acidity. Larger and more electronegative atoms can better stabilize the negative charge on the conjugate base, increasing the acidity of the conjugate acid.
Conclusion: A Nuance of Relative Strength
In summary, while weak bases do not have strong conjugate acids in the absolute sense, their conjugate acids are relatively stronger than the bases themselves. This relative strength is dictated by the equilibrium between the base and its conjugate acid, governed by the K<sub>b</sub> and K<sub>a</sub> values and the inherent relationship described by K<sub>w</sub>. Understanding this subtle but crucial difference is fundamental to grasping acid-base chemistry and its wide array of applications. The strength of the conjugate acid is relative and influenced by various factors, making it a dynamic aspect of chemical behavior rather than a simple binary classification. The principles discussed here are crucial for anyone studying or working in fields involving acid-base chemistry, from pharmaceuticals to environmental science.
Latest Posts
Latest Posts
-
What Are The Elements In Group 18 Called
Mar 29, 2025
-
How Do You Make A Standard Curve
Mar 29, 2025
-
Hydrogen Is A Metal Nonmetal Or Metalloid
Mar 29, 2025
-
Equation Of Tangent Line Implicit Differentiation
Mar 29, 2025
-
According To The Bronsted Lowry Definition
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Do Weak Bases Have Strong Conjugate Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
