Do You Always Use The Henderson Hasselbach For Titrations
Muz Play
Mar 17, 2025 · 5 min read
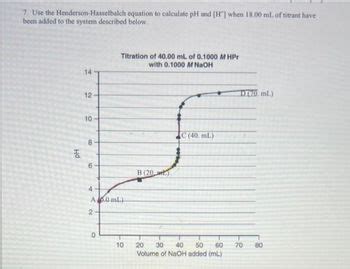
Table of Contents
Do You Always Use the Henderson-Hasselbalch Equation for Titrations?
The Henderson-Hasselbalch equation is a cornerstone of acid-base chemistry, providing a convenient way to calculate the pH of a buffer solution. But its application extends beyond simple buffer calculations. Many aspiring chemists and students often wonder about its role in titrations. While incredibly useful in certain titration contexts, it's not universally applicable throughout the entire titration process. This article delves into the nuances of using the Henderson-Hasselbalch equation during titrations, exploring when it's appropriate and when other methods are necessary.
Understanding the Henderson-Hasselbalch Equation
Before diving into its application in titrations, let's review the equation itself:
pH = pKa + log ([A⁻]/[HA])
Where:
- pH: The pH of the solution.
- pKa: The negative logarithm of the acid dissociation constant (Ka) of the weak acid.
- [A⁻]: The concentration of the conjugate base.
- [HA]: The concentration of the weak acid.
This equation is derived from the equilibrium expression for a weak acid and is most accurate when the following conditions are met:
- The solution is a buffer: Containing significant amounts of both a weak acid and its conjugate base.
- The concentration of the acid and its conjugate base are relatively high: This minimizes the impact of autoionization of water.
- The ionic strength is low: High ionic strength can affect activity coefficients, altering the accuracy of the equation.
The Henderson-Hasselbalch Equation's Role in Titrations
Titrations involve the gradual addition of a titrant (a solution of known concentration) to an analyte (a solution of unknown concentration) until the reaction is complete. In acid-base titrations, the pH changes dramatically near the equivalence point. The Henderson-Hasselbalch equation is most useful during the buffer region of a weak acid-strong base or weak base-strong acid titration.
The Buffer Region: Where the Henderson-Hasselbalch Equation Shines
During the buffer region, significant amounts of both the weak acid (or base) and its conjugate base (or acid) are present. This is precisely the condition where the Henderson-Hasselbalch equation excels. By knowing the initial concentration of the weak acid/base and the volume of titrant added, we can calculate the concentrations of the acid and its conjugate base at any point within the buffer region. Substituting these values into the equation accurately predicts the pH.
Example: Consider the titration of a weak acid, acetic acid (CH₃COOH), with a strong base, sodium hydroxide (NaOH). Before the equivalence point, the solution contains both acetic acid and its conjugate base, acetate (CH₃COO⁻). The Henderson-Hasselbalch equation allows us to calculate the pH at various points before the equivalence point. This is crucial for understanding the buffering capacity of the solution.
Limitations of the Henderson-Hasselbalch Equation in Titrations
While invaluable in the buffer region, the Henderson-Hasselbalch equation has limitations in other parts of a titration curve:
- Before the buffer region: At the beginning of the titration, when only the weak acid (or base) is present, the equation is not applicable. The pH is simply calculated using the initial concentration of the acid and its Ka value.
- At the equivalence point: At the equivalence point, the weak acid has completely reacted with the strong base (or vice versa). The Henderson-Hasselbalch equation is inappropriate here because the ratio [A⁻]/[HA] becomes undefined (or approaches infinity/zero). The pH at the equivalence point is determined by the hydrolysis of the conjugate base (or acid) and requires different calculations involving the Kb (or Ka) of the conjugate.
- Beyond the equivalence point: After the equivalence point, the pH is predominantly determined by the excess strong base (or acid) added. The Henderson-Hasselbalch equation is not relevant in this region; instead, the pH is simply calculated from the concentration of the excess strong base/acid.
- Polyprotic acids/bases: For titrations involving polyprotic acids or bases (those with multiple ionizable protons or hydroxide ions), the Henderson-Hasselbalch equation can be applied to each individual ionization step, but only within the appropriate buffer regions for each step.
Alternative Methods for pH Calculation in Titrations
The limitations highlighted above necessitate alternative approaches for pH calculation in specific titration stages:
- ICE tables: ICE (Initial, Change, Equilibrium) tables are a powerful tool for solving equilibrium problems, including pH calculations at various points in a titration. They are particularly useful before the buffer region and at the equivalence point for weak acid-strong base titrations.
- Using Kb or Ka expressions directly: For situations outside the buffer region, using the appropriate equilibrium constant expression (Ka or Kb) directly provides a more accurate pH calculation.
- Strong acid-strong base titrations: For strong acid-strong base titrations, the pH calculation is straightforward. The pH is simply determined by the concentration of excess H⁺ or OH⁻ ions, and the Henderson-Hasselbalch equation is unnecessary.
Practical Implications and Applications
Understanding when and when not to use the Henderson-Hasselbalch equation is crucial for accurate pH calculations during titrations. The inappropriate application of the equation can lead to significant errors in determining the equivalence point and the overall understanding of the titration process. This knowledge is essential for various fields, including:
- Analytical chemistry: Accurate pH calculations are fundamental to quantitative analysis, such as determining the concentration of unknown solutions.
- Biochemistry: Buffer solutions are vital in biological systems, and the Henderson-Hasselbalch equation aids in understanding pH regulation and enzyme activity.
- Environmental science: Acid-base titrations are often used to monitor water quality, and accurate pH calculations are critical for interpreting results.
- Pharmaceutical industry: Maintaining the appropriate pH is critical in drug formulation and delivery, requiring precise pH calculations during development.
Conclusion: A Balanced Approach to Titration Calculations
The Henderson-Hasselbalch equation is a valuable tool for calculating pH during the buffer region of a weak acid-strong base or weak base-strong acid titration. However, it's crucial to remember its limitations. Relying solely on the Henderson-Hasselbalch equation for all parts of a titration curve can lead to inaccurate results. A comprehensive understanding of acid-base equilibrium principles and the use of appropriate methods—such as ICE tables and direct application of equilibrium expressions—are necessary for accurate pH calculation throughout the entire titration process. A balanced approach that leverages the strengths of different techniques provides the most robust and reliable analysis of titration data. Mastering these techniques allows for a more thorough understanding of acid-base chemistry and its diverse applications.
Latest Posts
Latest Posts
-
Function Of A Stage On A Microscope
Mar 17, 2025
-
What Is The Net Gain Of Atp In Glycolysis
Mar 17, 2025
-
Which Is Bigger Tert Or Chlorine
Mar 17, 2025
-
A Supersaturated Solution Is One That
Mar 17, 2025
-
Transitive Closure For Binary Relation Definition
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Do You Always Use The Henderson Hasselbach For Titrations . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
