Does A Gas Have A Definite Shape And Volume
Muz Play
Mar 28, 2025 · 6 min read
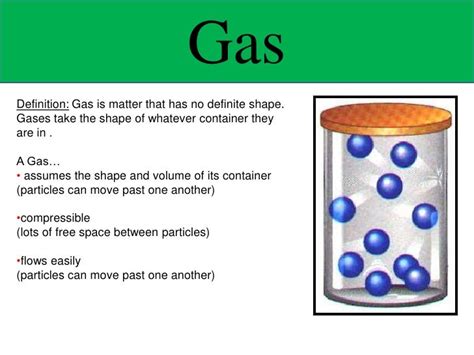
Table of Contents
Does a Gas Have a Definite Shape and Volume? Exploring the Properties of Gases
The question of whether a gas possesses a definite shape and volume is fundamental to understanding the nature of matter. Unlike solids and liquids, gases exhibit unique characteristics that define their behavior and interactions. This article delves into the properties of gases, explaining why they lack a definite shape and volume and exploring the scientific principles behind this phenomenon. We'll also examine the factors influencing gas behavior and their practical implications.
Understanding the States of Matter
Before diving into the specifics of gases, let's briefly review the three fundamental states of matter: solid, liquid, and gas. Each state is characterized by how its constituent particles (atoms or molecules) interact and are arranged:
-
Solids: Possess a definite shape and volume. Their particles are closely packed together in a fixed arrangement, held in place by strong intermolecular forces. This strong bonding restricts movement, leading to rigidity and a defined structure.
-
Liquids: Have a definite volume but take the shape of their container. Particles in a liquid are still relatively close together but have more freedom of movement than in a solid. This allows liquids to flow and adapt to the shape of their container while maintaining a constant volume.
-
Gases: Have neither a definite shape nor a definite volume. Particles in a gas are widely dispersed and move randomly at high speeds. The weak intermolecular forces allow the gas to expand to fill any container it occupies, making its shape and volume entirely dependent on the container's dimensions.
Why Gases Lack a Definite Shape and Volume
The key to understanding why gases lack a definite shape and volume lies in the nature of their intermolecular forces and the kinetic energy of their particles.
Weak Intermolecular Forces
Unlike solids and liquids, gases exhibit very weak intermolecular forces. These forces are the attractions between individual gas molecules. The weaker these forces, the less the molecules are constrained to a specific arrangement. In gases, the kinetic energy of the particles far outweighs the weak intermolecular forces, allowing the molecules to move freely and independently. This freedom of movement explains why gases readily expand to fill their containers.
High Kinetic Energy of Gas Particles
Gas particles possess high kinetic energy, meaning they are constantly in motion, moving randomly in all directions at high speeds. This high kinetic energy overcomes the weak intermolecular forces, further contributing to the lack of a defined shape and volume. The particles are not bound to fixed positions and readily disperse to occupy the available space. This random movement and the resulting collisions between particles and the container walls exert pressure.
Compressibility and Expandability
The lack of a fixed shape and volume in gases is also manifested in their compressibility and expandability. Gases can be easily compressed, meaning their volume can be significantly reduced by applying external pressure. This is because the particles are far apart, and there is considerable empty space between them. Conversely, gases readily expand to fill any available space. If the container is enlarged, the gas will expand to occupy the new, larger volume.
Factors Affecting Gas Behavior: The Ideal Gas Law
Several factors influence the behavior of gases, including:
-
Pressure (P): The force exerted by gas particles per unit area on the walls of their container. Pressure is typically measured in atmospheres (atm), Pascals (Pa), or millimeters of mercury (mmHg).
-
Volume (V): The amount of space occupied by the gas. Volume is usually measured in liters (L) or cubic meters (m³).
-
Temperature (T): A measure of the average kinetic energy of the gas particles. Temperature is expressed in Kelvin (K).
-
Amount of Gas (n): The number of moles of gas present. A mole is a unit representing a specific number of particles (Avogadro's number, approximately 6.022 x 10²³).
These four variables are related through the ideal gas law: PV = nRT, where R is the ideal gas constant. This law provides a simplified model for describing the behavior of gases under ideal conditions. However, it's important to remember that real gases may deviate from ideal behavior, especially at high pressures and low temperatures.
Deviations from Ideal Gas Behavior
The ideal gas law assumes that gas particles have negligible volume and do not interact with each other. These assumptions are not entirely accurate for real gases. At high pressures, the volume occupied by the gas particles themselves becomes significant compared to the total volume, leading to deviations from ideal behavior. Similarly, at low temperatures, intermolecular forces become more important, influencing the behavior of the gas. To account for these deviations, more complex equations of state, such as the van der Waals equation, are often used.
Applications and Real-World Examples
Understanding the properties of gases is crucial in numerous fields:
-
Meteorology: The behavior of gases in the atmosphere (e.g., pressure, temperature, humidity) is fundamental to weather forecasting and climate modeling.
-
Aerospace Engineering: The principles governing gas behavior are essential in designing aircraft, rockets, and spacecraft. The properties of gases in jet engines and rocket propulsion systems are critical for their operation.
-
Chemical Engineering: Gases are involved in many chemical processes, including combustion, synthesis, and separation. Understanding gas behavior is crucial for designing efficient and safe chemical reactors.
-
Medicine: Respiratory gases (oxygen and carbon dioxide) are vital for human life. Medical devices and treatments often rely on understanding gas properties and their behavior in the human body.
-
Environmental Science: The study of atmospheric gases and their interactions is critical for understanding air pollution and climate change. Understanding gas behavior plays a crucial role in developing strategies to mitigate these environmental challenges.
Conclusion: The Indefinite Nature of Gases
In summary, gases lack a definite shape and volume because their particles are widely dispersed, move randomly at high speeds, and are characterized by weak intermolecular forces. This freedom of movement allows gases to expand to fill their containers, leading to their indefinite shape. The high kinetic energy of their particles further contributes to their ability to readily adapt to changes in volume, hence their indefinite volume. While the ideal gas law provides a simplified model for understanding gas behavior, it's essential to acknowledge that real gases may deviate from ideal behavior under specific conditions. Understanding the properties and behavior of gases is crucial across numerous scientific and engineering disciplines, impacting areas ranging from weather forecasting to the development of advanced technologies. The indefinite nature of gases, a consequence of the interplay between kinetic energy and intermolecular forces, is a fundamental concept in our understanding of the physical world.
Latest Posts
Latest Posts
-
Electric Field Of A Charged Disk
Mar 31, 2025
-
5 Blind Man And The Elephant
Mar 31, 2025
-
Which Statement Describes The Citric Acid Cycle
Mar 31, 2025
-
Why Do Plants Love Water In Bio Terms
Mar 31, 2025
-
Identifying The Important Intermolecular Forces In Pure Compounds
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
