Does Gas Have A Fixed Shape
Muz Play
Mar 24, 2025 · 6 min read
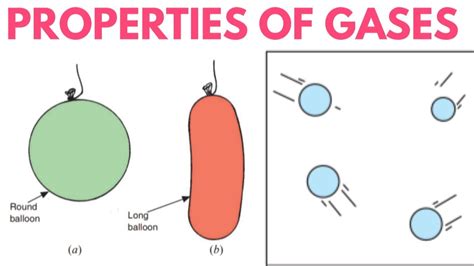
Table of Contents
Does Gas Have a Fixed Shape? Exploring the Properties of Gases
Gases are one of the four fundamental states of matter, alongside solids, liquids, and plasmas. Unlike solids and liquids, gases don't have a fixed shape or volume. This defining characteristic stems from the unique behavior of gas molecules and the forces that govern their interactions. This article delves deep into the properties of gases, explaining why they lack a fixed shape and exploring the concepts that underpin this behavior. We'll also examine the relationship between gas properties and factors like temperature and pressure, and finally, we'll discuss some real-world applications of understanding gas behavior.
Understanding the Kinetic Molecular Theory of Gases
The behavior of gases can be effectively explained using the kinetic molecular theory (KMT). This theory postulates that gases consist of a large number of tiny particles (atoms or molecules) that are in constant, random motion. These particles are incredibly small compared to the distances between them, meaning that the volume occupied by the particles themselves is negligible compared to the overall volume of the gas.
Key Postulates of the Kinetic Molecular Theory:
- Particles are in constant, random motion: Gas particles are constantly moving in straight lines until they collide with each other or the container walls. These collisions are elastic, meaning that kinetic energy is conserved.
- The volume of gas particles is negligible: The volume occupied by the gas particles themselves is insignificant compared to the total volume of the gas. This allows for significant compressibility.
- Interparticle forces are negligible: The attractive or repulsive forces between gas particles are weak and insignificant compared to their kinetic energy. This explains why gases expand to fill their containers.
- Collisions are elastic: When gas particles collide with each other or the container walls, no kinetic energy is lost.
- The average kinetic energy of gas particles is proportional to temperature: The temperature of a gas is directly related to the average kinetic energy of its particles. Higher temperatures mean faster-moving particles.
Why Gases Don't Have a Fixed Shape
The lack of a fixed shape in gases is a direct consequence of the postulates outlined in the KMT. Because gas particles are in constant, random motion and the forces between them are weak, they are not constrained to fixed positions. Instead, they move freely and independently, readily adapting to the shape of any container they occupy.
Imagine filling a balloon with air. The air molecules, initially concentrated in a smaller space, rapidly spread out to occupy the entire volume of the balloon. The balloon's shape becomes the shape of the gas because the gas molecules are free to move and fill the available space. If the balloon were then squeezed into a different shape, the gas molecules would quickly rearrange themselves to conform to the new shape. This illustrates the fundamental lack of a fixed shape characteristic of gases.
The Role of Interparticle Forces
While the KMT assumes negligible interparticle forces, it's important to acknowledge that these forces do exist, albeit weakly. These weak forces, known as van der Waals forces, can have a small effect on the behavior of real gases, particularly at lower temperatures and higher pressures. However, even in these cases, the dominant factor remains the kinetic energy of the gas particles, leading to the absence of a fixed shape.
The Influence of Temperature and Pressure on Gas Shape
Temperature and pressure significantly influence the behavior of gases. As temperature increases, the kinetic energy of the gas particles increases, causing them to move more rapidly and spread out further. This doesn't change the fact that they lack a fixed shape; rather, it amplifies their tendency to fill the available space.
Pressure, on the other hand, affects the volume occupied by the gas. Increasing pressure forces the gas particles closer together, reducing the volume but not fundamentally altering their lack of a defined shape. The gas particles still fill the available space, however small that space may become under compression.
Ideal Gas Law and Real Gases
The ideal gas law, PV = nRT, describes the relationship between pressure (P), volume (V), number of moles (n), temperature (T), and the ideal gas constant (R) for an ideal gas. An ideal gas is a hypothetical gas that perfectly obeys the ideal gas law. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, where intermolecular forces become more significant. However, the fundamental principle of gases lacking a fixed shape still applies, even for real gases. The deviations simply reflect the complexities introduced by intermolecular interactions.
Real-World Applications of Understanding Gas Behavior
Understanding the properties of gases and their lack of fixed shape is critical in many aspects of science, engineering, and everyday life. Here are just a few examples:
- Weather forecasting: Atmospheric gases, primarily nitrogen and oxygen, are constantly moving and interacting, driven by temperature and pressure gradients. Understanding their behavior is crucial for accurate weather prediction.
- Aerosol sprays: These rely on the ability of gases to expand and propel liquids or solids out of a container. The propellant gas lacks a fixed shape and pressure, making it perfect for the task.
- Balloons: The inflation of balloons demonstrates the ability of gases to expand and conform to the shape of their container. The gas (typically helium or air) fills the balloon's volume, taking its shape.
- Internal combustion engines: The expansion of gases during combustion drives the pistons in these engines, converting chemical energy into mechanical work. Understanding gas expansion is vital in engine design.
- Pneumatic systems: These systems utilize compressed air or other gases to power machinery or control processes. The compressibility and lack of fixed shape of gases make them ideal for such applications.
- Deep-sea diving: The behavior of gases under high pressure is critical for diver safety. Understanding how gases compress and expand with depth is essential for preventing decompression sickness.
- Industrial processes: Many industrial processes involve the use of gases, such as in chemical reactions, transportation of materials, and power generation. Understanding gas behavior is crucial for efficient and safe operations.
- Medical applications: Gases like oxygen are essential in medical treatments and life support systems. Their behavior, especially under controlled conditions, is important for effective medical interventions.
Conclusion: The Ever-Changing Shape of Gases
In conclusion, gases do not possess a fixed shape due to the fundamental properties of their constituent particles as described by the kinetic molecular theory. The constant, random motion of these particles, combined with negligible interparticle forces, allows gases to readily adapt to the shape of their container. While temperature and pressure influence the volume and density of gases, they do not alter their inherent lack of a defined shape. Understanding this fundamental property of gases is paramount in various scientific, engineering, and everyday applications, making it a cornerstone of numerous fields. The dynamic and ever-changing nature of gas shape highlights the fascinating complexity of the gaseous state of matter.
Latest Posts
Latest Posts
-
The Ph Of A Solution Is
Mar 27, 2025
-
Deutsch And Deutsch Late Selection Theory
Mar 27, 2025
-
How To Find Domain And Range Algebraically
Mar 27, 2025
-
Differences Between Sheep And Human Brain
Mar 27, 2025
-
How To Calculate Enthalpy Of Combustion
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Does Gas Have A Fixed Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
