Does Hydrochloric Acid Fully Dissociate In Water
Muz Play
Mar 16, 2025 · 5 min read
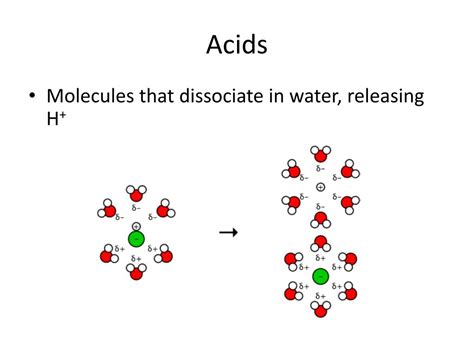
Table of Contents
Does Hydrochloric Acid Fully Dissociate in Water? A Deep Dive into Strong Acid Behavior
Hydrochloric acid (HCl), a strong acid, is frequently described as fully dissociating in water. This statement, while a useful simplification for many applications, requires a more nuanced understanding to truly appreciate its accuracy. This article delves into the intricacies of HCl dissociation, exploring the factors that influence its behavior and clarifying the limitations of the "fully dissociated" characterization.
Understanding Acid Dissociation
Before diving into the specifics of HCl, let's establish a foundational understanding of acid dissociation. Acids, by definition, donate protons (H⁺ ions) to a solution. The extent to which an acid donates these protons determines its strength. This process is represented by an equilibrium reaction:
HA ⇌ H⁺ + A⁻
Where:
- HA represents the acid molecule
- H⁺ represents the proton (hydrogen ion)
- A⁻ represents the conjugate base
The equilibrium constant, Kₐ, quantifies the strength of the acid. A higher Kₐ value indicates a stronger acid, meaning it dissociates more readily. Strong acids, like HCl, have very large Kₐ values, suggesting a high degree of dissociation. Conversely, weak acids have small Kₐ values, meaning they only partially dissociate.
The Case of Hydrochloric Acid (HCl)
HCl is a classic example of a strong acid. When HCl is added to water, it readily donates its proton to water molecules, forming hydronium ions (H₃O⁺) and chloride ions (Cl⁻):
HCl + H₂O ⇌ H₃O⁺ + Cl⁻
The equilibrium lies heavily to the right, meaning a significant majority of HCl molecules dissociate. This is often summarized as "complete dissociation," leading many to believe that all HCl molecules dissociate in water. However, this is a simplification.
The Reality of "Complete" Dissociation
While the vast majority of HCl molecules dissociate in dilute aqueous solutions, it's crucial to acknowledge that the dissociation is not truly 100%. Even strong acids like HCl retain a minuscule fraction of undissociated molecules. The equilibrium constant, although very large, is still finite. This means there will always be a small, but non-zero concentration of undissociated HCl molecules present.
The concentration of undissociated HCl is so small in dilute solutions that its impact on most calculations and applications is negligible. This is why the "fully dissociated" approximation is widely used. However, in highly concentrated solutions of HCl or under specific conditions, the concentration of undissociated HCl becomes more noticeable and cannot be ignored.
Factors Influencing HCl Dissociation
Several factors can influence the extent of HCl dissociation:
Concentration of HCl
As mentioned, the concentration of HCl significantly impacts the degree of dissociation. In dilute solutions, the approximation of complete dissociation holds well. However, in concentrated solutions, the activity coefficients of the ions change, and the equilibrium shifts slightly towards the undissociated HCl. The higher the concentration, the less "complete" the dissociation becomes. This is due to the increased ionic strength of the solution affecting the interaction between the ions and the undissociated HCl molecules.
Temperature
Temperature also plays a role. While the effect is relatively small for HCl, increasing the temperature can slightly increase the degree of dissociation. This is because the equilibrium constant, Kₐ, is temperature-dependent. Higher temperatures typically favor reactions that absorb heat (endothermic reactions), although the effect on HCl dissociation is relatively minor compared to the influence of concentration.
Solvent Effects
The nature of the solvent is crucial. While water is the typical solvent for HCl dissociation studies, using different solvents will drastically alter the behavior. In non-polar solvents, HCl dissociates much less readily. The dielectric constant of the solvent plays a key role; higher dielectric constants facilitate ion separation and dissociation.
Ionic Strength
The overall ionic strength of the solution impacts the activity coefficients of the ions (H₃O⁺ and Cl⁻). High ionic strength can suppress the dissociation of HCl, even in dilute solutions. This is related to the Debye-Hückel theory, which describes the effect of ionic strength on the activity of ions in solution.
Practical Implications of the "Incomplete" Dissociation
While the incomplete dissociation of HCl is usually insignificant, understanding its limitations is crucial in certain contexts:
- Precise Calculations: In highly precise calculations, particularly involving concentrated HCl solutions or non-aqueous solutions, the slight deviation from complete dissociation needs to be taken into account. Activity coefficients must be incorporated into calculations to obtain accurate results.
- Spectroscopic Studies: Spectroscopic techniques can detect even small concentrations of undissociated HCl. These measurements can provide valuable insights into the actual extent of dissociation under various conditions.
- Theoretical Chemistry: Theoretical modeling of chemical reactions often needs to account for the incomplete dissociation of strong acids for a more accurate representation of the system.
Beyond the Simple Model
The notion that HCl fully dissociates in water is a convenient approximation for introductory chemistry and many practical applications. However, a deeper understanding reveals that the reality is more nuanced. The degree of dissociation is never truly 100%, although it's exceptionally close in dilute solutions. Understanding the factors affecting the dissociation – concentration, temperature, solvent, and ionic strength – is crucial for a comprehensive understanding of HCl's behavior in aqueous solutions and beyond. This knowledge is not just for theoretical exploration; it has real-world implications in precise calculations, advanced research, and the development of accurate models in various scientific fields.
Conclusion: A More Accurate Perspective
In summary, while the "fully dissociated" model of HCl in water is a useful simplification for many purposes, it's not entirely accurate. The dissociation is extremely high in dilute solutions, but a minuscule fraction of HCl molecules remains undissociated. Recognizing this subtle nuance leads to a more accurate understanding of the behavior of strong acids and provides a more rigorous foundation for various chemical calculations and interpretations. Understanding the influence of factors like concentration, temperature, and solvent allows for a more complete and accurate picture of this fundamental chemical process. This comprehensive perspective is not merely academic; it holds practical implications for various scientific and engineering disciplines.
Latest Posts
Latest Posts
-
The Concentration Of A Solution Can Be Expressed In
Mar 17, 2025
-
How To Write A Mass Balance Equation
Mar 17, 2025
-
Using E Z Designators Identify The Configuration
Mar 17, 2025
-
Differences Between Eukaryotic And Prokaryotic Gene Expression
Mar 17, 2025
-
Understanding The Definitions Of Ionization Energy And Electron Affinity
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Hydrochloric Acid Fully Dissociate In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
