Does Increasing Pressure Of A Gas Decrease Entroppy
Muz Play
Mar 15, 2025 · 6 min read
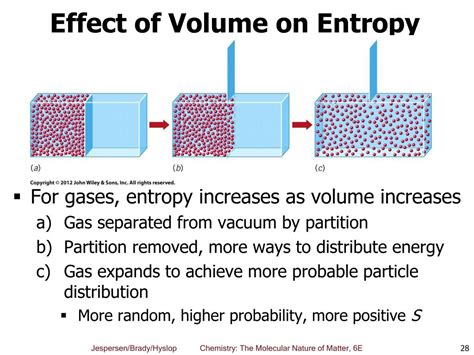
Table of Contents
Does Increasing the Pressure of a Gas Decrease Entropy?
The relationship between pressure and entropy in gases is a fundamental concept in thermodynamics. Intuitively, it might seem counterintuitive that increasing the pressure of a gas would decrease its entropy. After all, we often associate higher pressure with more disordered systems. However, a deeper understanding reveals a more nuanced picture. This article delves into the complexities of this relationship, exploring the microscopic behavior of gas molecules and the macroscopic implications of pressure changes on entropy.
Understanding Entropy: A Measure of Disorder
Before diving into the pressure-entropy relationship, it's crucial to define entropy. Entropy (S) is a thermodynamic property that quantifies the degree of disorder or randomness within a system. A system with high entropy is highly disordered, while a system with low entropy is highly ordered. At a microscopic level, entropy is related to the number of possible microstates (arrangements of molecules) that correspond to a given macrostate (observable properties like temperature, pressure, and volume). The more microstates consistent with a given macrostate, the higher the entropy.
Key Factors Influencing Entropy:
- Temperature: Higher temperatures generally lead to higher entropy, as increased kinetic energy allows molecules to explore a wider range of possible states.
- Volume: A larger volume provides more space for molecules to move around, leading to increased disorder and higher entropy.
- Number of Particles: More particles increase the number of possible microstates, thus increasing entropy.
- Pressure: The relationship between pressure and entropy is more complex and is the focus of this article.
The Pressure-Entropy Relationship in Ideal Gases
For an ideal gas (a theoretical gas that obeys certain simplifying assumptions), the relationship between pressure and entropy can be understood through the ideal gas law (PV = nRT) and the thermodynamic definition of entropy.
The Ideal Gas Law and Its Implications:
The ideal gas law connects pressure (P), volume (V), number of moles (n), gas constant (R), and absolute temperature (T). If we increase the pressure of an ideal gas while keeping the temperature constant (isothermal process), the volume must decrease. This decrease in volume directly impacts the entropy.
Isothermal Compression: Decreasing Entropy
During an isothermal compression, the temperature remains constant. Reducing the volume restricts the movement of gas molecules, leading to a decrease in the number of possible microstates and thus a decrease in entropy. The molecules become more confined, resulting in a more ordered state. The decrease in volume directly reduces the number of available positions for the particles, leading to lower entropy. This is a crucial point: while increasing pressure itself doesn't directly dictate entropy, the consequential volume reduction is the primary driver of the entropy decrease in isothermal processes.
Adiabatic Compression: A More Complex Scenario
An adiabatic process occurs without heat exchange with the surroundings. When an ideal gas undergoes adiabatic compression, the pressure increases, and the volume decreases. Unlike isothermal compression, the temperature also increases during adiabatic compression. This increase in temperature partially offsets the entropy decrease caused by the volume reduction.
The entropy change in an adiabatic process is given by:
ΔS = nCv ln(T2/T1)
where:
- ΔS is the change in entropy
- n is the number of moles
- Cv is the molar heat capacity at constant volume
- T1 and T2 are the initial and final temperatures, respectively.
While the volume decrease contributes to a reduction in entropy, the temperature increase contributes to an increase in entropy. The net effect depends on the magnitude of these competing factors. In many cases, the entropy change during adiabatic compression is still negative, albeit less significant than in isothermal compression.
Beyond Ideal Gases: Real Gases and Intermolecular Forces
The analysis above focuses on ideal gases. Real gases, however, deviate from ideal behavior due to intermolecular forces (attractive and repulsive forces between gas molecules). These forces play a crucial role in influencing the pressure-entropy relationship.
Intermolecular Forces and Entropy:
At low pressures, real gases behave similarly to ideal gases. However, as pressure increases, the intermolecular forces become more significant. Attractive forces can lead to a slight increase in order, thereby reducing entropy. This effect adds to the entropy decrease caused by the volume reduction. Repulsive forces, on the other hand, can contribute to increased disorder and hence a higher entropy, partially counteracting the effect of the reduced volume.
The Role of Molecular Size:
The size of gas molecules also affects entropy. Larger molecules occupy more volume, leading to a slightly higher entropy at the same pressure and temperature compared to smaller molecules.
Illustrative Example: Isothermal Compression of a Gas
Let's consider a simple example to visualize the effect. Imagine a gas contained in a cylinder fitted with a piston. If we isothermally compress the gas by pushing the piston inward, the volume decreases, and the pressure increases. The molecules have less space to move around; their positions are more restricted, leading to a more ordered state and consequently, a decrease in entropy.
Experimental Evidence and Applications
The theoretical predictions about the decrease in entropy upon increasing pressure in gases are supported by experimental observations. Techniques like calorimetry and spectroscopic measurements can be used to determine entropy changes during compression processes.
The understanding of pressure-entropy relationships has significant implications in various applications, including:
- Refrigeration and Air Conditioning: Compression and expansion of refrigerants are fundamental processes in these systems, and entropy changes play a crucial role in determining efficiency.
- Chemical Engineering: Many industrial processes involve gas compression and expansion, where precise control of entropy is vital for optimizing energy consumption and product yield.
- Power Generation: Power plants often utilize gas turbines where the expansion and compression of gases are key processes affecting the overall efficiency.
- Meteorology: Understanding entropy changes in atmospheric gases is crucial for modeling weather patterns and climate change.
Conclusion: The Nuances of Pressure and Entropy
Increasing the pressure of a gas, particularly under isothermal conditions, typically leads to a decrease in entropy. This decrease is primarily attributed to the reduction in volume, restricting the movement of gas molecules and resulting in a more ordered state. While adiabatic compression presents a more complex scenario with the temperature change influencing entropy, the volume reduction often remains a dominant factor. The behavior of real gases deviates slightly from ideal gases due to intermolecular forces and molecular size, but the general trend of entropy reduction with increasing pressure largely holds true. A thorough understanding of the interplay between pressure, volume, temperature, and intermolecular forces is crucial for predicting and controlling entropy changes in gaseous systems across various scientific and engineering applications. Further research into the specific behaviour of different gases under varied conditions continues to refine our understanding of this crucial thermodynamic relationship.
Latest Posts
Latest Posts
-
Can You Be In Love With Two People
Mar 17, 2025
-
Factors That Influence The Elasticity Of Supply
Mar 17, 2025
-
Describe How The Atoms In A Compound Are Held Together
Mar 17, 2025
-
How Is Absorbance Linked To Rate Of Reaction
Mar 17, 2025
-
What Happens To Electrons In An Ionic Bond
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Increasing Pressure Of A Gas Decrease Entroppy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
