How Is Absorbance Linked To Rate Of Reaction
Muz Play
Mar 17, 2025 · 6 min read
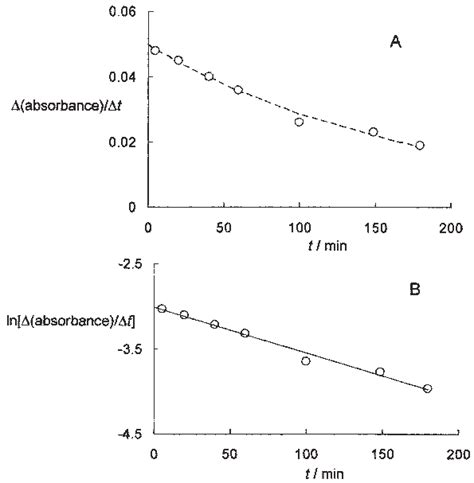
Table of Contents
How is Absorbance Linked to Rate of Reaction?
Understanding the relationship between absorbance and the rate of reaction is crucial in numerous fields, from chemistry and biochemistry to environmental science and pharmacology. Many chemical reactions, especially those involving colored species, can be monitored effectively by measuring the change in absorbance over time. This technique, known as spectrophotometry, allows for the quantitative study of reaction kinetics. This article will delve into the intricacies of this link, exploring the underlying principles, practical applications, and potential limitations.
The Beer-Lambert Law: The Foundation of Spectrophotometric Analysis
The cornerstone of understanding how absorbance relates to reaction rate is the Beer-Lambert Law. This law states that the absorbance (A) of a solution is directly proportional to the concentration (c) of the absorbing species and the path length (l) of the light through the solution. Mathematically, it's represented as:
A = εlc
where:
- A is the absorbance (unitless)
- ε is the molar absorptivity (L mol⁻¹ cm⁻¹), a constant specific to the absorbing species and the wavelength of light used.
- l is the path length (typically in cm), the distance the light travels through the solution.
- c is the concentration of the absorbing species (typically in mol L⁻¹).
This law is fundamental because it establishes a direct relationship between the measurable quantity (absorbance) and the concentration of a reactant or product. In a reaction where the concentration of a colored species changes over time, the absorbance will also change proportionally, providing a means to monitor the reaction progress.
Monitoring Reaction Rates using Absorbance
The change in absorbance over time (ΔA/Δt) is directly proportional to the rate of the reaction if the absorbing species is directly involved. This makes absorbance a powerful tool for kinetic studies. Consider a simple reaction:
A → B
where reactant A is colored and product B is colorless or absorbs light at a different wavelength. As the reaction proceeds, the concentration of A decreases, and consequently, its absorbance decreases. By measuring the absorbance at regular intervals, we can determine the rate of disappearance of A. Conversely, if B is colored, its increasing absorbance can be used to monitor the reaction rate.
Determining Reaction Order Using Absorbance Data
The relationship between absorbance and reaction rate can be used to determine the reaction order with respect to a specific reactant. For example, if a reaction is first-order with respect to reactant A, a plot of ln(A) versus time will yield a straight line with a slope equal to -k (the rate constant). Since A is directly proportional to the concentration of A (from the Beer-Lambert Law), a plot of ln(Absorbance) versus time can also provide the reaction order and rate constant. Similarly, other reaction orders can be determined by analyzing the appropriate plots of absorbance data.
Practical Applications: A Wide Range of Uses
The link between absorbance and reaction rate finds application across numerous scientific disciplines:
-
Enzyme Kinetics: The activity of enzymes can be monitored by measuring the absorbance of a substrate or product. This is particularly useful in determining Michaelis-Menten constants (Km) and maximum reaction velocities (Vmax), crucial parameters in understanding enzyme behavior.
-
Pharmaceutical Analysis: Drug degradation or metabolism can be followed by monitoring absorbance changes over time. This is essential for determining drug shelf life and understanding metabolic pathways.
-
Environmental Monitoring: The rate of degradation of pollutants in water or soil samples can be monitored using spectrophotometry, providing insights into environmental remediation strategies.
-
Chemical Kinetics: The study of reaction mechanisms and rate laws is greatly facilitated by using absorbance measurements to track reactant and product concentrations.
-
Industrial Processes: Real-time monitoring of industrial chemical processes using absorbance measurements allows for better process control and optimization.
Factors Affecting Absorbance Measurements and Their Implications on Reaction Rate Determination
Several factors can affect the accuracy and reliability of absorbance measurements, thereby influencing the determination of reaction rates. These factors need careful consideration:
-
Temperature: Temperature changes can alter the equilibrium constant of a reaction and affect the molar absorptivity of the absorbing species. Maintaining a constant temperature throughout the experiment is crucial.
-
Solvent Effects: The solvent used can influence the absorbance of the species involved in the reaction. Using the same solvent throughout the experiment is essential.
-
Wavelength Selection: The choice of wavelength for absorbance measurement is crucial. The wavelength should be chosen where the absorbing species has maximum absorbance to enhance sensitivity and minimize interference from other species.
-
Stray Light: Stray light entering the spectrophotometer can lead to inaccurate absorbance readings. Regular calibration and maintenance of the spectrophotometer are necessary.
-
Cuvette Selection: The type of cuvette (e.g., quartz, glass, plastic) used can affect absorbance measurements. Using clean and matched cuvettes is important for consistent results.
Limitations of Using Absorbance to Determine Reaction Rates
While absorbance measurements are a powerful technique for determining reaction rates, there are some limitations to consider:
-
Non-linearity: The Beer-Lambert Law is only valid under certain conditions. At high concentrations, deviations from linearity can occur, affecting the accuracy of absorbance-based rate determinations.
-
Interfering Species: If other species in the reaction mixture absorb light at the same wavelength as the species being monitored, this can lead to inaccurate absorbance measurements.
-
Slow Reactions: For very slow reactions, the time required for accurate measurements might be impractical.
-
Complex Reactions: In complex reactions with multiple steps, interpreting absorbance data to determine the rate of a specific step can be challenging.
Advanced Techniques and Considerations
Beyond simple absorbance measurements, more advanced techniques can enhance the accuracy and information obtained from reaction rate studies:
-
Stopped-flow spectrophotometry: This technique is particularly useful for studying very fast reactions by rapidly mixing reactants and then monitoring the absorbance change.
-
Temperature-jump techniques: These methods perturb the equilibrium of a reaction by rapidly changing the temperature and then monitor the return to equilibrium using absorbance measurements.
-
Data analysis techniques: Sophisticated data analysis methods, such as non-linear regression, are often used to fit absorbance data to kinetic models and determine rate constants accurately.
Conclusion
The relationship between absorbance and reaction rate, as governed by the Beer-Lambert Law, provides a powerful and versatile technique for studying chemical kinetics. Spectrophotometry offers a simple, readily accessible, and cost-effective method for monitoring reaction progress and determining reaction orders. However, it’s essential to be aware of the factors affecting absorbance measurements and the limitations of the technique. By carefully considering these factors and employing appropriate experimental designs and data analysis methods, researchers can leverage the power of absorbance measurements to gain valuable insights into the rates and mechanisms of a wide range of chemical and biochemical reactions. The continued development of advanced spectrophotometric techniques further enhances the capabilities of this fundamental analytical tool.
Latest Posts
Latest Posts
-
A Shaft Of A Long Bone Is Called
Mar 17, 2025
-
According To The Bronsted Lowry Definition A Base Is
Mar 17, 2025
-
Is A R2nh In A Ring A Good Leaving Group
Mar 17, 2025
-
Find The Shape Of Quadrilateral Given Vertex Coordinates
Mar 17, 2025
-
Can Hydrophilic Molecules Pass Through Membrane
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Is Absorbance Linked To Rate Of Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
