Is A R2nh In A Ring A Good Leaving Group
Muz Play
Mar 17, 2025 · 5 min read
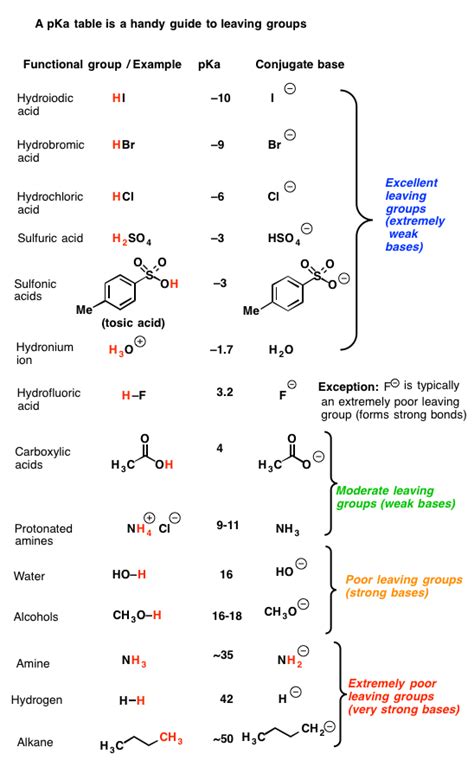
Table of Contents
Is an R2NH in a Ring a Good Leaving Group? A Comprehensive Examination
Leaving groups play a crucial role in organic chemistry reactions, particularly in nucleophilic substitution (SN1 and SN2) and elimination reactions (E1 and E2). Understanding the characteristics of a good leaving group is essential for predicting reaction outcomes and designing synthetic strategies. This article delves into the question of whether a secondary amine (R2NH) within a ring structure constitutes a good leaving group. We'll explore the factors influencing leaving group ability and analyze the specific challenges and possibilities presented by cyclic secondary amines.
Understanding Leaving Groups: Key Characteristics
A good leaving group is one that can readily accept a pair of electrons upon departure from a molecule. This ability is largely determined by several key factors:
1. Stability of the Leaving Group:
A stable leaving group is inherently more favorable. Highly stable anions, like those that are resonance-stabilized or can be further stabilized through inductive effects, are excellent leaving groups. The more stable the conjugate base formed after the leaving group departs, the easier it is for the reaction to proceed.
2. Basicity of the Leaving Group:
There's an inverse relationship between the basicity of a leaving group and its ability to leave. Weak bases are better leaving groups because they are less likely to attract and regain the departing proton. Strong bases, on the other hand, hold onto the electrons tightly, making them poor leaving groups.
3. Polarizability of the Leaving Group:
A more polarizable leaving group facilitates the departure process. Polarizability enhances the ability of the leaving group to dissipate the negative charge developed during departure. This can significantly affect the rate of SN2 reactions.
4. Steric Effects:
Steric hindrance can significantly impact the ease of departure. Bulky leaving groups may experience steric clashes, hindering their departure and thus slowing down the reaction rate.
R2NH as a Leaving Group: The General Case
In the general case, where R2NH is not part of a ring system, it is a very poor leaving group. Secondary amines are relatively strong bases, meaning they have a high affinity for protons and are reluctant to depart with a positive charge. The resulting amide ion (R2N-) is highly unstable and will readily try to regain a proton.
Consider a typical SN2 reaction: The nucleophile would need to attack the carbon atom bearing the R2NH group, with the R2NH subsequently departing as R2N-. The high basicity and instability of R2N- make this process highly unfavorable.
R2NH in a Ring: The Cyclical Influence
When the secondary amine (R2NH) is incorporated into a ring system, the situation becomes more nuanced. The ring structure can impose constraints that affect the leaving group's behavior in several ways:
1. Ring Strain:
If the ring is strained (e.g., a three or four-membered ring), the release of ring strain upon the departure of the leaving group can contribute to driving the reaction forward. This additional driving force can partially compensate for the poor leaving group nature of R2NH. The relief of ring strain adds a significant energetic favorability to the overall reaction.
2. Conformational Restrictions:
The cyclic structure imposes specific conformational restrictions. These restrictions can influence the orientation of the leaving group and the nucleophile, affecting the stereochemistry and reaction rate of SN2 reactions. For instance, the ring's conformation could make the backside attack by a nucleophile more or less favorable.
3. Electronic Effects:
The ring structure may also have electronic effects on the R2NH group. Electron-withdrawing substituents on the ring can reduce the basicity of the nitrogen atom, potentially making it a slightly better leaving group. Conversely, electron-donating substituents would further enhance the basicity, worsening its departure characteristics.
4. Specific Ring Systems:
The size and type of ring structure are crucial. Five and six-membered rings, being less strained than smaller rings, might not see the same degree of beneficial strain relief upon leaving group departure. The effect will likely be less pronounced in these cases.
Making R2NH in a Ring a Better Leaving Group: Strategies
While R2NH in a ring is inherently a poor leaving group, there are strategies to improve its departure capabilities:
1. Quaternization:
Converting the secondary amine to a quaternary ammonium salt significantly improves its leaving group ability. The quaternary ammonium ion (R3N+R') is a much weaker base and can more readily depart as a neutral species. This approach transforms a poor leaving group into a reasonably good one.
2. Protonation:
Protonating the nitrogen atom can increase the leaving group's ability. The protonated amine (R2NH2+) is a much better leaving group than the neutral form due to its reduced basicity. The positive charge helps in stabilizing the departure process.
3. Conversion to a Better Leaving Group:
Transforming the R2NH group into another better leaving group entirely can provide a more efficient reaction pathway. This could involve a series of reaction steps that replace the R2NH with a more suitable leaving group such as a tosylate (-OTs), mesylate (-OMs), or triflate (-OTf).
4. Reaction Conditions:
Optimizing reaction conditions such as solvent choice, temperature, and the concentration of reagents can influence the reaction rate, even with a poor leaving group. High temperatures can sometimes overcome the energy barrier associated with a poor leaving group.
Conclusion: Context is Key
Ultimately, whether an R2NH group in a ring is a “good” leaving group depends heavily on the specific context: the size of the ring, the presence of substituents, and the nature of the reaction being considered. While inherently a poor leaving group, certain circumstances, like significant ring strain or the application of strategies to improve its characteristics, can increase the likelihood of successful departure. It is vital to carefully evaluate these factors before assuming its suitability in a reaction design. Understanding the intricacies of leaving group behavior allows for strategic reaction planning and ultimately successful synthetic outcomes. The general principle remains that, without specific modifications, R2NH remains a poor leaving group due to its strong basicity and instability upon departure.
Latest Posts
Latest Posts
-
Does Gas Have A Definite Shape
Mar 17, 2025
-
If The Equilibrium Constant Is Negative What Does That Mean
Mar 17, 2025
-
How Does An Atom Become A Cation
Mar 17, 2025
-
Which Body Cavity Protects The Spinal Column
Mar 17, 2025
-
Sampling Distribution Of The Sample Mean Calculator
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is A R2nh In A Ring A Good Leaving Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
