What Happens To Electrons In An Ionic Bond
Muz Play
Mar 17, 2025 · 6 min read
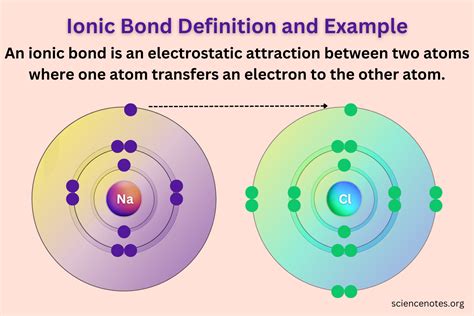
Table of Contents
What Happens to Electrons in an Ionic Bond? A Deep Dive
Ionic bonds, a cornerstone of chemistry, represent a fundamental type of chemical bonding characterized by the electrostatic attraction between oppositely charged ions. Understanding what happens to electrons in this process is crucial to grasping the nature of these bonds and their implications in various chemical phenomena. This article will delve into the intricacies of electron transfer in ionic bonding, exploring the underlying principles, examples, and exceptions.
The Electron Transfer Process: A Tale of Two Atoms
At the heart of ionic bonding lies the transfer of electrons from one atom to another. This transfer isn't a gentle nudge; it's a decisive shift in electron ownership, resulting in the formation of charged particles called ions. The process typically involves a metal atom, which readily loses electrons, and a non-metal atom, which readily gains electrons.
This asymmetry in electron affinity stems from the differing electronegativities of the atoms involved. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Metals, generally possessing low electronegativity, are more willing to relinquish their valence electrons, while non-metals, possessing high electronegativity, have a strong pull on electrons.
Let's visualize this with a simple example: the formation of sodium chloride (NaCl), common table salt.
Sodium (Na) and Chlorine (Cl): A Perfect Ionic Match
Sodium, an alkali metal, has one valence electron loosely held in its outermost shell. Chlorine, a halogen, has seven valence electrons, needing only one more to complete its outermost shell. The electronegativity difference between sodium and chlorine is substantial. Chlorine's stronger electronegativity allows it to "steal" the single valence electron from sodium.
This electron transfer is not a gradual process; it's a relatively abrupt event resulting in:
- A positively charged sodium ion (Na⁺): Sodium, having lost one negatively charged electron, now possesses one more proton than electrons, acquiring a positive charge. This positively charged ion is called a cation.
- A negatively charged chloride ion (Cl⁻): Chlorine, having gained one electron, now possesses one more electron than protons, acquiring a negative charge. This negatively charged ion is called an anion.
These oppositely charged ions are then held together by a powerful electrostatic force of attraction, forming the ionic bond in NaCl. This electrostatic attraction is significantly stronger than the weak intermolecular forces present in other types of molecules.
The Role of Electron Configuration and Octet Rule
The driving force behind electron transfer in ionic bonding is the tendency of atoms to achieve a stable electron configuration, typically resembling that of a noble gas. This is often referred to as the octet rule, stating that atoms tend to gain, lose, or share electrons to achieve eight electrons in their outermost shell (valence shell). Exceptions exist, particularly for elements in the first and second rows of the periodic table, but the octet rule provides a useful framework for understanding ionic bonding.
In our NaCl example:
- Sodium, by losing its single valence electron, achieves the stable electron configuration of neon (Ne), a noble gas with a filled outermost shell.
- Chlorine, by gaining one electron, achieves the stable electron configuration of argon (Ar), another noble gas with a filled outermost shell.
This attainment of stable electron configurations is energetically favorable, leading to the formation of the ionic bond. The resulting ionic compound is more stable than the individual atoms.
Beyond Sodium Chloride: Exploring Diverse Ionic Compounds
The principles outlined above apply broadly to various ionic compounds. Let's consider some further examples:
Magnesium Oxide (MgO)
Magnesium (Mg) has two valence electrons, while oxygen (O) has six. Magnesium loses two electrons to become Mg²⁺, achieving the neon electron configuration, while oxygen gains two electrons to become O²⁻, achieving the neon electron configuration as well. The resulting 2+ and 2- charges balance each other, leading to the formation of MgO.
Calcium Chloride (CaCl₂)
Calcium (Ca) has two valence electrons, while chlorine (Cl) has seven. Calcium loses two electrons to become Ca²⁺, achieving the argon electron configuration. Each chlorine atom gains one electron to become Cl⁻, achieving the argon configuration. Therefore, two chlorine atoms are needed to accept the two electrons from one calcium atom, forming CaCl₂.
Factors Influencing Ionic Bond Formation
Several factors influence the likelihood of ionic bond formation:
- Electronegativity Difference: A large electronegativity difference between the atoms involved is crucial. The greater the difference, the more likely the electron transfer and consequent ionic bond formation.
- Ionization Energy: The ionization energy is the energy required to remove an electron from an atom. Metals with low ionization energies readily lose electrons, favoring ionic bond formation.
- Electron Affinity: Electron affinity is the energy change that occurs when an atom gains an electron. Nonmetals with high electron affinities strongly attract electrons, further promoting ionic bond formation.
- Lattice Energy: Once ions are formed, they arrange themselves into a stable crystalline lattice structure. The lattice energy, the energy released upon formation of this lattice, is a significant factor in the overall stability of the ionic compound. A high lattice energy indicates a strong ionic bond.
Exceptions and Limitations of the Simple Model
While the simple model of electron transfer provides a good understanding of ionic bonding, it has limitations:
- Polar Covalent Bonds: In some cases, the electronegativity difference between atoms isn't large enough for a complete electron transfer. Instead, a polar covalent bond forms, where electrons are shared unequally, resulting in partial charges. The boundary between ionic and polar covalent bonding is not always sharply defined.
- Complex Ions: Many ionic compounds involve complex ions, which are groups of atoms carrying an overall charge. For example, in ammonium chloride (NH₄Cl), the ammonium ion (NH₄⁺) is a polyatomic cation formed through covalent bonding, but it behaves as a single unit in the ionic interaction with chloride ions.
- Metallic Bonding: Metals exhibit a different type of bonding, called metallic bonding, where valence electrons are delocalized across a lattice of metal atoms. This contrasts with the localized electron transfer in ionic bonding.
Applications of Ionic Compounds
Ionic compounds find widespread applications in various fields:
- Medicine: Many salts are essential electrolytes in the body, playing crucial roles in nerve impulse transmission, muscle contraction, and fluid balance.
- Industry: Ionic compounds are used in diverse industrial processes, including manufacturing fertilizers, cleaning agents, and construction materials.
- Everyday Life: Table salt (NaCl), baking soda (NaHCO₃), and gypsum (CaSO₄·2H₂O) are just a few examples of ionic compounds found commonly in our daily lives.
Conclusion: A Powerful Force in Chemistry
Ionic bonding, driven by the transfer of electrons between atoms with differing electronegativities, is a fundamental force in chemistry. Understanding this process, including the roles of electron configuration, octet rule, and lattice energy, provides a crucial foundation for comprehending the properties and behavior of a vast array of chemical substances. While the simple model of complete electron transfer has limitations, it remains a valuable tool for understanding this important aspect of chemical bonding. Further exploration into the complexities of ionic interactions, including polar covalent bonds and complex ions, opens avenues for a deeper understanding of chemical phenomena.
Latest Posts
Latest Posts
-
What Type Of Symmetry Do Sponges Have
Mar 17, 2025
-
Find The Roots Of A Complex Number
Mar 17, 2025
-
After An Enzyme Reaction Is Completed The Enzyme
Mar 17, 2025
-
List The Functions Of Proteins In The Text Area Below
Mar 17, 2025
-
Subatomic Particle With A Negative Charge
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Happens To Electrons In An Ionic Bond . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
