Does Increasing Temperature Increase Reaction Rate
Muz Play
Mar 27, 2025 · 5 min read
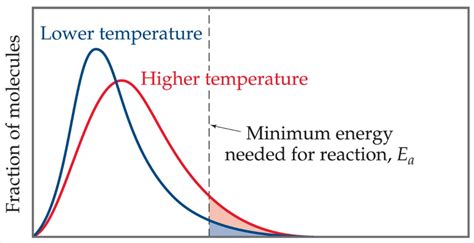
Table of Contents
Does Increasing Temperature Increase Reaction Rate? A Deep Dive into Reaction Kinetics
The relationship between temperature and reaction rate is a cornerstone of chemistry, impacting everything from industrial processes to biological functions. The simple answer is a resounding yes, increasing temperature generally increases the reaction rate. However, understanding why this happens requires a deeper dive into the principles of reaction kinetics and the underlying mechanisms driving chemical transformations. This article will explore this fundamental concept, examining the theoretical basis, practical implications, and exceptions to this rule.
The Collision Theory: A Foundation for Understanding Temperature's Influence
At the heart of understanding the temperature-reaction rate relationship lies the collision theory. This theory postulates that for a reaction to occur, reactant molecules must collide with sufficient energy and the correct orientation. Let's break this down:
1. The Energy Requirement: Activation Energy (Ea)
Every chemical reaction possesses an activation energy (Ea) – a minimum energy barrier that colliding molecules must overcome to initiate the reaction. This energy is needed to break existing bonds within the reactant molecules, allowing the formation of new bonds and the creation of products.
2. The Orientation Requirement: Successful Collisions
Even with sufficient energy, a collision might not result in a reaction if the molecules don't collide with the correct orientation. Imagine trying to fit two jigsaw pieces together – they need to align perfectly. Similarly, reactant molecules need to approach each other in a specific way for bonds to break and reform effectively.
Temperature's Role: Increasing the Kinetic Energy of Molecules
Temperature is a measure of the average kinetic energy of molecules. Increasing the temperature boosts the kinetic energy of the molecules, causing them to move faster and collide more frequently. This increased frequency of collisions directly translates into a higher chance of successful collisions, i.e., collisions that possess both sufficient energy to surpass the activation energy and the correct orientation.
The Arrhenius Equation: Quantifying the Temperature-Rate Relationship
The quantitative relationship between temperature and reaction rate is elegantly captured by the Arrhenius equation:
k = A * e^(-Ea/RT)
Where:
- k is the rate constant (a measure of reaction speed).
- A is the pre-exponential factor (related to the frequency and orientation of collisions).
- Ea is the activation energy.
- R is the ideal gas constant.
- T is the absolute temperature (in Kelvin).
This equation shows that the rate constant (and therefore the reaction rate) is exponentially dependent on temperature. A small increase in temperature can lead to a significant increase in the reaction rate, especially for reactions with high activation energies.
Visualizing the Effect: The Maxwell-Boltzmann Distribution
The Maxwell-Boltzmann distribution provides a visual representation of how temperature affects the distribution of molecular energies. This distribution shows that at higher temperatures, a larger fraction of molecules possesses energy greater than or equal to the activation energy (Ea). This means more molecules have the necessary energy to react upon collision, leading to a faster reaction rate.
Practical Applications and Examples: Where Temperature Matters
The impact of temperature on reaction rates is ubiquitous across various fields:
1. Industrial Chemistry: Optimizing Reaction Conditions
In industrial settings, controlling temperature is crucial for optimizing reaction rates and yields. Many industrial processes are designed to operate at elevated temperatures to accelerate reactions and improve efficiency. Examples include:
- Haber-Bosch process (Ammonia synthesis): This process requires high temperatures and pressures to synthesize ammonia from nitrogen and hydrogen.
- Petroleum refining: Cracking and reforming of petroleum fractions involve high-temperature reactions to produce valuable products like gasoline and other fuels.
- Polymerization reactions: Many polymerization reactions, such as the production of plastics, are conducted at elevated temperatures to achieve desired rates of polymerization.
2. Food Science and Cooking: Speeding up Chemical Changes
Cooking involves numerous chemical reactions, and temperature plays a vital role in determining cooking times and outcomes. Higher temperatures accelerate reactions like the browning of meat (Maillard reaction) and the softening of vegetables.
3. Biology and Biochemistry: Enzyme Activity and Metabolic Rates
Biological systems are exquisitely sensitive to temperature changes. Enzyme activity, a crucial aspect of metabolic processes, is strongly influenced by temperature. While increasing temperature initially increases enzyme activity, excessively high temperatures can denature enzymes, leading to a dramatic decrease in reaction rates and potentially cell death. This explains why high fevers can be dangerous.
4. Environmental Science: Decomposition Rates and Climate Change
Temperature affects decomposition rates in ecosystems. Higher temperatures accelerate the breakdown of organic matter, impacting nutrient cycling and greenhouse gas emissions. Climate change, with its associated temperature increases, significantly alters these processes, potentially disrupting ecosystems' balance.
Exceptions and Complications: When the Rule Doesn't Always Apply
While the general rule holds true, there are exceptions and complexities to consider:
- Reactions with very low activation energies: For reactions with extremely low activation energies, the temperature dependence may be less pronounced.
- Reactions involving multiple steps: In multi-step reactions, the overall rate may not be solely determined by the temperature dependence of a single step.
- Competing reactions: If a temperature increase favors an undesired side reaction more than the desired one, the overall yield of the desired product might decrease even though the rate of the desired reaction increases.
- Catalyst effects: Catalysts can significantly lower the activation energy of a reaction, making it less sensitive to temperature changes.
Conclusion: A Fundamental Principle with Broad Implications
The relationship between temperature and reaction rate is a fundamental principle in chemistry with far-reaching consequences across diverse fields. While generally, increasing temperature leads to faster reactions, understanding the underlying mechanisms, particularly the collision theory and the Arrhenius equation, is vital for accurately predicting and controlling reaction rates. The nuances of multi-step reactions, catalyst effects, and competing reactions highlight the complexities involved, requiring a case-by-case analysis to fully grasp the impact of temperature on any specific reaction. The ability to manipulate and control reaction rates through temperature adjustment remains a cornerstone of chemical engineering, biological research, and many other scientific disciplines.
Latest Posts
Latest Posts
-
Where Does Respiration Take Place In Eukaryotic Cells
Mar 30, 2025
-
Prove The Fundamental Theorem Of Arithmetic
Mar 30, 2025
-
Draw The Shear And Moment Diagrams For The Cantilever Beam
Mar 30, 2025
-
Calculus Early Transcendentals By James Stewart
Mar 30, 2025
-
Cauchy Schwarz Inequality For Complex Numbers
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Does Increasing Temperature Increase Reaction Rate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
