Does Positive Reduction Potential Sum Mean Spontaneous
Muz Play
Mar 15, 2025 · 5 min read
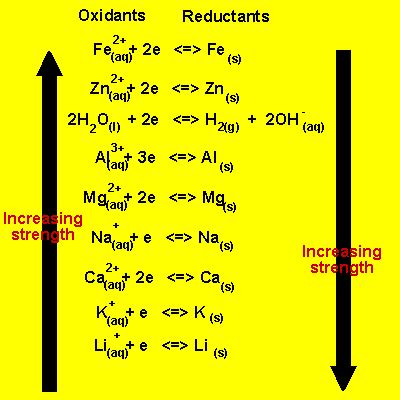
Table of Contents
Does a Positive Reduction Potential Sum Mean Spontaneous? Understanding Redox Reactions and Gibbs Free Energy
The question of whether a positive reduction potential sum indicates a spontaneous reaction is a fundamental concept in electrochemistry. While a positive cell potential (E°<sub>cell</sub>) strongly suggests spontaneity, the relationship isn't always straightforward. This article delves into the intricacies of redox reactions, reduction potentials, Gibbs Free Energy, and the conditions under which a positive reduction potential sum reliably predicts spontaneity.
Understanding Reduction Potentials and Electrochemical Cells
Reduction potential (E°) measures a substance's tendency to gain electrons and undergo reduction. A higher positive reduction potential indicates a stronger tendency to be reduced. Electrochemical cells harness these tendencies:
- Oxidation: Loss of electrons. Occurs at the anode (negative electrode).
- Reduction: Gain of electrons. Occurs at the cathode (positive electrode).
An electrochemical cell comprises two half-cells: one where oxidation occurs and one where reduction occurs. The cell potential (E°<sub>cell</sub>) is the difference between the reduction potentials of the two half-reactions:
E°<sub>cell</sub> = E°<sub>cathode</sub> - E°<sub>anode</sub>
A positive E°<sub>cell</sub> traditionally signifies a spontaneous reaction under standard conditions (298K, 1 atm, 1M concentration). A negative E°<sub>cell</sub> indicates a non-spontaneous reaction under standard conditions; energy input is required to drive the reaction forward.
Standard Reduction Potentials: A Reference Point
Standard reduction potentials are measured relative to the standard hydrogen electrode (SHE), which is arbitrarily assigned a potential of 0.00 V. All other reduction potentials are then measured relative to this benchmark. A table of standard reduction potentials provides a valuable tool for predicting the spontaneity of redox reactions.
The Crucial Role of Gibbs Free Energy (ΔG)
While the cell potential provides a convenient indicator of spontaneity, the true thermodynamic measure is the Gibbs Free Energy change (ΔG). ΔG represents the maximum amount of reversible work a system can perform at constant temperature and pressure. The relationship between ΔG and E°<sub>cell</sub> is:
ΔG = -nFE°<sub>cell</sub>
Where:
- ΔG is the Gibbs Free Energy change (in Joules)
- n is the number of moles of electrons transferred in the balanced redox reaction
- F is Faraday's constant (96485 C/mol)
- E°<sub>cell</sub> is the standard cell potential (in Volts)
This equation reveals the direct link:
- Positive E°<sub>cell</sub> → Negative ΔG → Spontaneous reaction
- Negative E°<sub>cell</sub> → Positive ΔG → Non-spontaneous reaction
Therefore, a positive sum of reduction potentials, leading to a positive E°<sub>cell</sub>, directly translates to a negative ΔG, confirming spontaneity under standard conditions.
Non-Standard Conditions: The Nernst Equation
The above relationships hold true only under standard conditions. In real-world scenarios, concentrations and temperatures deviate from standard values. The Nernst equation accounts for these deviations:
E<sub>cell</sub> = E°<sub>cell</sub> - (RT/nF)lnQ
Where:
- E<sub>cell</sub> is the cell potential under non-standard conditions
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature (in Kelvin)
- Q is the reaction quotient
The Nernst equation demonstrates that changes in concentration and temperature can alter the cell potential and, consequently, the spontaneity of the reaction. A positive E°<sub>cell</sub> doesn't guarantee a positive E<sub>cell</sub> under non-standard conditions.
When a Positive Reduction Potential Sum Might be Misleading
While a positive E°<sub>cell</sub> is a strong indicator of spontaneity, it's crucial to acknowledge limitations:
-
Kinetic Factors: Even with a positive E°<sub>cell</sub>, the reaction might be kinetically hindered. The activation energy might be too high for the reaction to proceed at a noticeable rate. Catalysts can often overcome this limitation.
-
Competing Reactions: In complex systems, multiple redox reactions might occur simultaneously. A positive E°<sub>cell</sub> for a particular reaction doesn't guarantee it will dominate over other competing reactions with potentially more favorable kinetics.
-
Side Reactions: Unexpected side reactions could consume reactants or interfere with the main redox process, affecting the overall spontaneity.
Practical Applications and Examples
Understanding the relationship between reduction potentials and spontaneity is crucial in various applications:
-
Battery Design: Designing effective batteries relies on selecting electrode materials with appropriate reduction potentials to ensure sufficient voltage and spontaneity for efficient energy storage and release.
-
Corrosion Prevention: Understanding the reduction potentials of metals helps predict their susceptibility to corrosion. Protective coatings or cathodic protection techniques are employed to prevent spontaneous oxidation (corrosion) of metals.
-
Electroplating: Electroplating uses controlled redox reactions to deposit a thin layer of metal onto another surface. The choice of metal and electrolyte solution is dictated by the desired reduction potential for the plating process.
-
Analytical Chemistry: Redox titrations are used to determine the concentration of unknown substances by carefully monitoring the cell potential during the titration. The equivalence point is often indicated by a significant change in cell potential.
Beyond Standard Potentials: A Deeper Dive
The concept of standard reduction potentials provides a valuable framework, but it's essential to consider the limitations and the influence of non-standard conditions. The Nernst equation and a deeper understanding of thermodynamics are crucial for accurate predictions of reaction spontaneity in real-world scenarios.
Moreover, the intricacies of electron transfer mechanisms and the role of catalysts are equally important in understanding the overall rate and feasibility of a redox reaction.
Conclusion: A Holistic Perspective
A positive sum of reduction potentials, resulting in a positive E°<sub>cell</sub>, strongly suggests a spontaneous reaction under standard conditions. However, this is just a starting point. A thorough understanding of Gibbs Free Energy, the Nernst equation, and the influence of kinetic and other factors is essential for accurately predicting the spontaneity of a redox reaction under diverse circumstances. The interplay between thermodynamics and kinetics determines the feasibility and rate of the reaction in practical applications. It's a holistic perspective that combines theoretical understanding with practical considerations that allows for accurate and reliable predictions. Remember that while a positive E°<sub>cell</sub> is a good indicator, it’s not a guarantee of spontaneity, and always consider the context of the reaction under study.
Latest Posts
Latest Posts
-
If The Finches On The Galapagos Islands
Mar 15, 2025
-
How To Find A Perpendicular Vector
Mar 15, 2025
-
How Could Sulfur Form An Ion
Mar 15, 2025
-
What Elemsnts Are Most Likey To Turn Into Anions Why
Mar 15, 2025
-
What Is The Difference Between Hunger And Appetite
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Does Positive Reduction Potential Sum Mean Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
