How Could Sulfur Form An Ion
Muz Play
Mar 15, 2025 · 5 min read
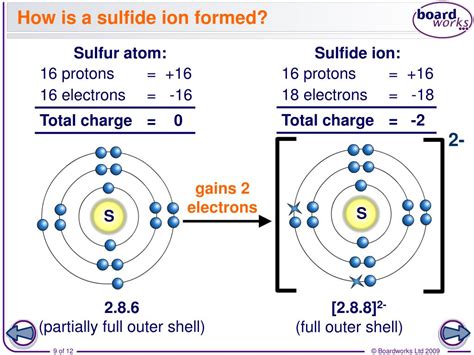
Table of Contents
How Could Sulfur Form an Ion? Delving into the Chemistry of Sulfur Ions
Sulfur, a vibrant yellow nonmetal, plays a crucial role in various biological and industrial processes. Understanding its ability to form ions is fundamental to comprehending its diverse chemical behavior. This comprehensive article explores the intricacies of sulfur ion formation, covering its electronic structure, oxidation states, and the different types of ions it can produce. We'll also delve into the factors influencing ion formation and the practical applications stemming from these ionic interactions.
Understanding Sulfur's Electronic Structure
To understand how sulfur forms ions, we must first examine its electronic configuration. Sulfur (S) has an atomic number of 16, meaning it possesses 16 protons and 16 electrons in its neutral state. Its electronic configuration is 1s²2s²2p⁶3s²3p⁴. This configuration indicates that the outermost shell (the valence shell) contains six electrons. Atoms strive for stability, often achieved by having a full outermost shell, typically containing eight electrons (the octet rule). This inherent drive for stability dictates sulfur's behavior in ion formation.
The Octet Rule and Sulfur's Reactivity
The octet rule governs the reactivity of many elements, including sulfur. To achieve a stable octet, sulfur can either gain two electrons to complete its outermost shell or lose six electrons to empty its outermost shell. However, losing six electrons requires a significant amount of energy, making it less energetically favorable. Therefore, sulfur is more likely to gain two electrons to form an anion.
Sulfur's Common Ions: Sulfide and Other Anions
The most common ion sulfur forms is the sulfide ion (S²⁻). By gaining two electrons, sulfur achieves a stable noble gas configuration similar to argon (Ar). This negatively charged ion is a crucial component in many inorganic compounds and plays a vital role in various chemical reactions.
Beyond Sulfide: Exploring Other Oxidation States
While the sulfide ion (S²⁻) is the most prevalent, sulfur's versatility extends to other oxidation states, resulting in a range of different anions. The oxidation state refers to the hypothetical charge an atom would have if all its bonds were completely ionic. Sulfur can exhibit oxidation states ranging from -2 (in sulfide) to +6 (in sulfate). These varying oxidation states lead to the formation of several significant sulfur-containing anions, including:
-
Sulfide (S²⁻): The most common anion, formed by gaining two electrons. Found in various metal sulfides, such as iron sulfide (FeS) and lead sulfide (PbS).
-
Disulfide (S₂²⁻): Contains two sulfur atoms bonded together, each with a -1 charge. This ion is found in some metal disulfide compounds and plays a crucial role in protein structure through disulfide bridges.
-
Sulfite (SO₃²⁻): Contains one sulfur atom bonded to three oxygen atoms, carrying a -2 charge. A crucial component in many chemical processes and an intermediate in sulfur oxidation.
-
Sulfate (SO₄²⁻): Contains one sulfur atom bonded to four oxygen atoms, carrying a -2 charge. A very stable and common anion found extensively in nature and industrial applications.
-
Thiosulfate (S₂O₃²⁻): Contains two sulfur atoms and three oxygen atoms, carrying a -2 charge. Used in photography as a fixing agent and in medical applications.
Each of these anions displays unique chemical properties and reactivity, influencing their applications in various fields. The oxidation state of sulfur significantly dictates the properties and behavior of these ions.
Factors Influencing Sulfur Ion Formation
Several factors influence the formation of sulfur ions:
-
Electronegativity: Sulfur's electronegativity (its ability to attract electrons in a chemical bond) plays a significant role. When sulfur bonds with a more electronegative element, such as oxygen, it tends to lose electrons (higher positive oxidation state). Conversely, when it bonds with a less electronegative element, such as a metal, it tends to gain electrons (negative oxidation state).
-
Ionic Radius: The size of the ion also influences its stability. The sulfide ion (S²⁻) has a larger ionic radius compared to the neutral sulfur atom, due to the addition of two electrons. This increased size can affect its reactivity and interactions with other ions.
-
Environmental Conditions: The reaction environment significantly impacts sulfur's ability to form ions. Factors such as temperature, pressure, and the presence of other reactants can influence the type and amount of ions formed. For example, in aqueous solutions, sulfur can form various oxoanions depending on the pH and the oxidizing/reducing conditions.
-
Type of Reaction: The nature of the chemical reaction involving sulfur determines the resulting ion. In redox (reduction-oxidation) reactions, sulfur can undergo changes in its oxidation state, leading to the formation of different ions.
The Significance of Sulfur Ions
Sulfur ions are essential components in numerous areas:
Biological Significance
-
Protein Structure: Disulfide bonds (S-S) formed between cysteine residues in proteins are crucial for their three-dimensional structure and function.
-
Enzymes: Many enzymes contain sulfur in their active sites, contributing to their catalytic activity.
-
Metabolism: Sulfur plays a vital role in various metabolic pathways in living organisms.
Industrial Applications
-
Fertilizers: Sulfates are essential components of many fertilizers, supplying sulfur to plants.
-
Mining: Metal sulfides are abundant in various ores and play a significant role in metallurgy.
-
Chemical Industry: Sulfuric acid (H₂SO₄), derived from sulfur, is a cornerstone of numerous industrial processes.
-
Paper Production: Sulfates are used in the Kraft process for paper pulp production.
Environmental Impact
-
Acid Rain: Sulfur dioxide (SO₂) emissions from industrial processes and volcanic activity contribute significantly to acid rain.
-
Air Pollution: Sulfur oxides are major air pollutants, impacting human health and the environment.
-
Ocean Acidification: The absorption of sulfur dioxide into the oceans can contribute to ocean acidification.
Conclusion: A Versatile Element with Diverse Ionic Forms
Sulfur's ability to form a variety of ions demonstrates its remarkable versatility as a chemical element. The understanding of its electronic structure, the factors governing ion formation, and the diverse applications of sulfur ions remains vital for advancements in chemistry, biology, and environmental science. From the formation of stable sulfide minerals to the intricate disulfide bridges in proteins, the role of sulfur ions is fundamental across numerous scientific disciplines. Further research and exploration into the behavior of sulfur ions promise even greater insights into its diverse roles and potential applications in the future.
Latest Posts
Latest Posts
-
Columns Of The Periodic Table Are Called
Mar 15, 2025
-
Is Boiling Point Intensive Or Extensive
Mar 15, 2025
-
A Triple Bond Is Generally Composed Of
Mar 15, 2025
-
Actin Filaments Are Anchored To Structures Called
Mar 15, 2025
-
How To Identify A Weak Acid
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Could Sulfur Form An Ion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
