Does Slt Water Break Down Rocks Or Compact Them
Muz Play
Mar 16, 2025 · 6 min read
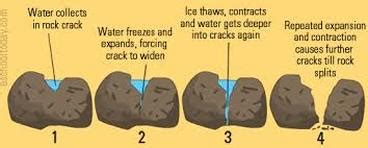
Table of Contents
Does Salt Water Break Down Rocks or Compact Them? The Complex Relationship Between Salinity and Lithification
The interaction between saltwater and rocks is a multifaceted process with significant geological implications. The common perception often simplifies this relationship, but the reality is far more nuanced. Salt water doesn't simply "break down" or "compact" rocks; its effect depends on a complex interplay of factors including the type of rock, the salinity of the water, the temperature, pressure, and the presence of other chemicals. This article delves into the intricate mechanisms by which saltwater influences rock formations, exploring both destructive and constructive processes.
The Destructive Power of Salt Water: Weathering and Erosion
Salt water contributes significantly to rock breakdown through various weathering and erosion processes. These processes are primarily driven by the chemical properties of salt and its interaction with the minerals comprising the rock.
1. Haloclasty: The Power of Crystallization
Haloclasty, also known as salt weathering, is a significant destructive force. This process involves the crystallization of salt within the pores and cracks of rocks. As water evaporates, salts precipitate, forming crystals that exert immense pressure on the surrounding rock. This pressure can lead to:
-
Fracturing: The expansion of salt crystals generates stresses that exceed the rock's tensile strength, causing it to fracture and disintegrate. This is particularly effective in porous rocks like sandstone and limestone.
-
Exfoliation: Repeated cycles of salt crystallization and dissolution can cause the outer layers of rocks to peel off, a process known as exfoliation. This is commonly observed in arid and semi-arid regions with high salinity.
-
Granular disintegration: The repetitive expansion and contraction of salt crystals within the rock matrix gradually weakens the bonds between individual grains, leading to granular disintegration and the eventual breakdown of the rock into smaller particles.
The effectiveness of haloclasty depends on factors like:
-
Salt concentration: Higher salt concentrations lead to more significant crystal growth and, thus, greater destructive power.
-
Rock porosity and permeability: Rocks with high porosity and permeability are more susceptible to salt intrusion and subsequent crystallization.
-
Climate: Arid and semi-arid climates with high evaporation rates are ideal for haloclasty, as they promote frequent salt crystallization cycles.
2. Chemical Weathering: Dissolution and Hydrolysis
Salt water doesn't only cause physical disintegration; it also participates in chemical weathering processes.
-
Dissolution: Some minerals, like calcite (the primary component of limestone and marble), are readily soluble in slightly acidic saltwater. This dissolution weakens the rock structure, making it more vulnerable to erosion. The presence of dissolved carbon dioxide in the water further enhances the dissolution process.
-
Hydrolysis: Hydrolysis is a chemical reaction between water and minerals in the rock, leading to their alteration and weakening. Salt ions in the water can catalyze these reactions, accelerating the rate of hydrolysis and increasing the susceptibility of rocks to weathering.
3. Abrasion and Erosion:
The movement of saltwater, especially in waves and currents, can act as a powerful erosive force. Saltwater carries suspended sediment particles that act as abrasives, impacting and wearing down rock surfaces. This mechanical erosion is particularly effective in coastal areas exposed to strong wave action.
The Constructive Role of Salt Water: Diagenesis and Lithification
While saltwater contributes significantly to rock breakdown, it also plays a crucial role in the formation of new rocks through diagenesis and lithification.
1. Cementation:
Dissolved salts in saltwater can act as cementing agents during diagenesis. As seawater percolates through sediment, dissolved salts precipitate out, binding sediment particles together. This process contributes to the formation of sedimentary rocks such as sandstones and conglomerates. The type of cementing agent (e.g., calcite, silica, iron oxides) influences the final properties of the rock. Evaporite deposits, formed through the evaporation of saltwater, are prime examples of rocks formed through the direct precipitation of salts.
2. Salt Diapirism:
In certain geological settings, large accumulations of salt can form diapirs – upwardly mobile masses of salt that pierce overlying rock layers. This process can significantly alter the structure and composition of surrounding rocks. While this process can lead to fracturing and deformation, it also creates unique geological features and contributes to the formation of new rock structures.
3. Influence on Pore Water Chemistry:
The chemistry of pore water within sediments and rocks is profoundly influenced by the salinity of the surrounding water. The presence of specific ions in saltwater can influence the rate of mineral reactions and contribute to the overall stability of the rock formation. For example, high salinity can inhibit the dissolution of certain minerals.
Factors Influencing the Outcome: A Complex Interplay
The ultimate impact of saltwater on rocks is not a simple binary of "breakdown" or "compaction." The outcome is a complex interplay of numerous factors:
-
Rock type: The mineralogy and structure of the rock determine its susceptibility to various weathering processes and its capacity to act as a reservoir for salts. Porous and permeable rocks are generally more susceptible to salt weathering.
-
Salinity: The concentration of salts in the water directly affects the intensity of haloclasty and chemical weathering.
-
Temperature: Temperature affects the solubility of salts and the rate of chemical reactions, influencing the overall weathering process.
-
Pressure: Pressure influences the rate of crystallization and the strength of rocks, impacting the effectiveness of haloclasty.
-
Water chemistry: The presence of other dissolved substances in the saltwater, besides salts, can alter the chemical reactions and influence the outcome.
Specific Examples: Case Studies in Rock-Saltwater Interactions
Numerous geological formations around the world showcase the complex interplay between saltwater and rocks.
-
Coastal cliffs: Coastal cliffs in many regions, like those along the Mediterranean Sea or the Pacific Northwest, are constantly being eroded by wave action and salt weathering, demonstrating the destructive power of saltwater.
-
Evaporite deposits: Massive salt deposits, such as those found in the Permian Basin of Texas and New Mexico, exemplify the constructive role of saltwater in forming sedimentary rocks through evaporation.
-
Subsurface reservoirs: In many sedimentary basins, saltwater plays a vital role in diagenesis, influencing the porosity and permeability of subsurface reservoirs, which are crucial for oil and gas exploration.
-
Salt diapirs: Regions with salt diapirs, such as the Gulf Coast of the United States, showcase the transformative power of salt movement on the structure and composition of surrounding rocks.
Conclusion: A Dynamic and Interdependent Relationship
The interaction between saltwater and rocks is a dynamic and multifaceted process. Saltwater can be a powerful agent of rock destruction through haloclasty, chemical weathering, and abrasion. Conversely, it can also play a constructive role in cementation, diagenesis, and the formation of unique geological structures. The ultimate impact of saltwater on rocks depends on a complex interplay of factors, making each geological setting unique and demanding a nuanced understanding of the processes involved. Further research into the intricate mechanisms governing rock-saltwater interactions is essential for a comprehensive understanding of geological processes and for managing the challenges associated with coastal erosion, groundwater resources, and subsurface resource management.
Latest Posts
Latest Posts
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
-
According To Dalton Atoms Of Different Elements Will Be
Mar 17, 2025
-
Examples Of Essential And Nonessential Nutrients
Mar 17, 2025
-
Electric Potential From A Point Charge
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does Slt Water Break Down Rocks Or Compact Them . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
