Does The Temperature Of Water Rise While It Is Boiling
Muz Play
Mar 16, 2025 · 5 min read
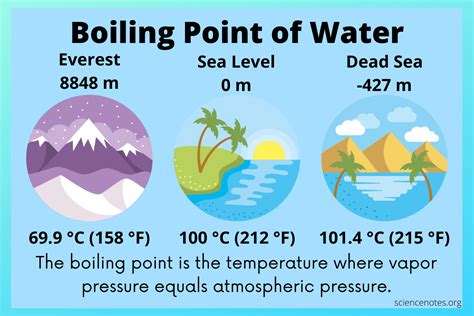
Table of Contents
Does the Temperature of Water Rise While it is Boiling?
The seemingly simple question, "Does the temperature of water rise while it is boiling?" leads to a fascinating exploration of the physics of phase transitions and the properties of water. While the intuitive answer might seem to be "no," the reality is more nuanced and offers valuable insights into the behavior of matter. This article delves into the intricacies of boiling, exploring the concepts of heat, temperature, and latent heat, to provide a comprehensive understanding of what happens to water's temperature during this crucial phase change.
Understanding Boiling: A Phase Transition
Boiling is a phase transition, specifically a change from the liquid phase to the gaseous phase (vaporization). This transition doesn't happen instantaneously; it's a process governed by the absorption of energy. Heat is the transfer of energy from a hotter object to a colder object. Temperature, on the other hand, is a measure of the average kinetic energy of the molecules in a substance. The molecules in boiling water are constantly moving, and their average kinetic energy is directly related to the water's temperature.
The Role of Heat Energy
For water to boil, it needs to absorb a significant amount of heat energy. This energy isn't simply used to increase the temperature; a large portion is used to overcome the intermolecular forces holding the water molecules together in the liquid state. These forces need to be broken to allow the molecules to escape into the gaseous phase as steam. This energy is known as the latent heat of vaporization.
Latent Heat of Vaporization: The Key to Understanding Boiling
The latent heat of vaporization is the amount of heat required to change one gram of a substance from a liquid to a gas at its boiling point without changing its temperature. For water, this value is remarkably high (approximately 2260 J/g or 540 cal/g). This high value explains why boiling water takes considerable time and energy. It's crucial to understand that while the water is boiling, this energy is being used to overcome the intermolecular forces, not to increase the temperature.
What Happens at the Boiling Point?
At standard atmospheric pressure (1 atm), water boils at 100°C (212°F). This is the temperature at which the vapor pressure of water equals the atmospheric pressure. Vapor pressure is the pressure exerted by the water molecules escaping from the liquid's surface. When this pressure is high enough to overcome the external pressure, bubbles of water vapor can form within the liquid and rise to the surface, resulting in the characteristic bubbling associated with boiling.
Constant Temperature During Boiling
During active boiling, the temperature of the water remains relatively constant at 100°C (at standard atmospheric pressure). This is because all the added heat energy is being used to convert liquid water into water vapor. Adding more heat will simply increase the rate of boiling; it won't raise the temperature of the water itself. This constant temperature is a defining characteristic of a phase transition.
Factors Affecting Boiling Point
It's important to note that the boiling point of water is not always 100°C. Several factors can influence it:
-
Altitude: At higher altitudes, the atmospheric pressure is lower, meaning the water will boil at a lower temperature. This is because the vapor pressure needs to overcome less external pressure.
-
Dissolved impurities: The presence of dissolved salts or other substances in the water can slightly elevate the boiling point. This is known as boiling point elevation.
-
Pressure: Increasing the pressure on the water increases its boiling point, while decreasing the pressure lowers it. This principle is used in pressure cookers, which cook food faster at higher temperatures and pressures.
The Myth of Rising Temperature During Boiling
The misconception that the temperature of water rises while boiling stems from a misunderstanding of the energy transfer involved. While heat is continuously added, it's not causing a temperature increase in the water itself. Instead, it's fueling the phase transition by breaking the intermolecular bonds and allowing the molecules to escape as steam. Therefore, the statement "the temperature of water rises while it is boiling" is fundamentally incorrect under standard conditions.
Implications and Applications
Understanding the constant temperature during boiling has significant implications across various fields:
-
Cooking: The constant boiling point allows for precise cooking techniques. Knowing that the water temperature remains at 100°C allows cooks to accurately control cooking times and temperatures.
-
Steam generation: Steam generation relies on the efficient conversion of liquid water to steam at a constant temperature. This process is crucial in power plants, industrial processes, and even everyday appliances like steam irons.
-
Scientific experiments: The predictable boiling point of water makes it an ideal substance for various scientific experiments, especially those involving calibration and temperature control.
-
Climate and weather: Understanding the phase transitions of water, including boiling, is crucial for understanding weather patterns, climate change, and the hydrological cycle.
Conclusion: Boiling, Heat, and Temperature – A Clear Distinction
In conclusion, the temperature of water does not rise while it is actively boiling under standard atmospheric pressure. The heat energy supplied is entirely consumed in overcoming the intermolecular forces to convert the liquid into a gas. This constant temperature at the boiling point is a fundamental concept in thermodynamics and has significant practical applications in various fields. Understanding the distinction between heat and temperature, and the role of latent heat, is crucial to grasping the complexities of this seemingly simple process. The myth of rising temperature during boiling highlights the importance of clarifying basic scientific concepts for better comprehension and application. This consistent temperature during the boiling process underlines the fundamental principles of phase transitions and underscores the vital role that heat energy plays in transforming matter from one state to another.
Latest Posts
Latest Posts
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Does The Temperature Of Water Rise While It Is Boiling . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
