Effective Nuclear Charge Zeff Is Defined As
Muz Play
Mar 22, 2025 · 6 min read
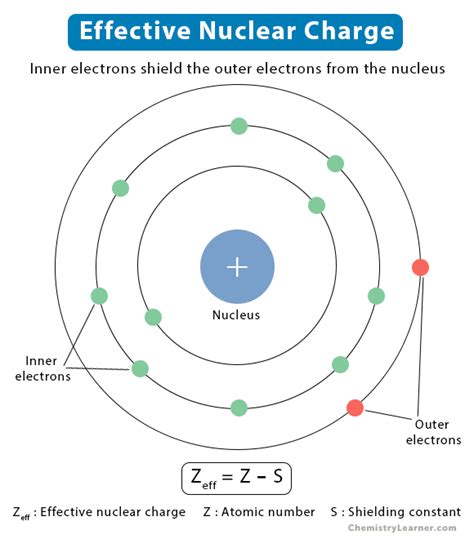
Table of Contents
Effective Nuclear Charge (Z<sub>eff</sub>): A Deep Dive into Atomic Structure and Properties
Effective nuclear charge (Z<sub>eff</sub>), also known as the effective core charge, is a crucial concept in chemistry that describes the net positive charge experienced by an electron in a multi-electron atom. Understanding Z<sub>eff</sub> is fundamental to explaining various atomic properties like atomic size, ionization energy, and electronegativity. This comprehensive guide will delve into the definition, calculation, trends, and applications of effective nuclear charge.
Defining Effective Nuclear Charge
In a hydrogen atom, with only one proton and one electron, the attractive force between the nucleus and the electron is directly proportional to the nuclear charge (+1). However, in multi-electron atoms, things become more complex. The electrons don't just interact with the nucleus; they also interact with each other. This electron-electron repulsion shields the outer electrons from the full positive charge of the nucleus. Effective nuclear charge (Z<sub>eff</sub>) represents the net positive charge experienced by a specific electron after accounting for this shielding effect. It's the difference between the actual nuclear charge (Z, the number of protons) and the shielding effect (S) provided by other electrons:
Z<sub>eff</sub> = Z - S
Where:
- Z is the atomic number (number of protons)
- S is the shielding constant (a measure of electron-electron repulsion)
Calculating Effective Nuclear Charge: Methods and Approximations
Precisely calculating the shielding constant (S) is computationally challenging for atoms with many electrons. However, several methods offer reasonable approximations:
Slater's Rules: A Simple Approach
Slater's rules provide a relatively straightforward way to estimate the shielding constant. This method assigns different weighting factors to electrons based on their location relative to the electron of interest. The rules are:
-
Write the electron configuration of the atom in the following form: (1s)(2s, 2p)(3s, 3p)(3d)(4s, 4p) and so on.
-
Electrons in the same group as the electron of interest contribute 0.35 (0.30 for 1s electrons).
-
Electrons in the principal energy level (n) just below the electron of interest contribute 0.85.
-
All electrons in lower principal energy levels (n-2 or lower) contribute 1.00.
-
For d and f electrons, the shielding from other electrons in the same n level is 0.35, and the shielding from all electrons in lower levels is 1.00.
Example: Let's calculate Z<sub>eff</sub> for a 3p electron in chlorine (Cl). Chlorine has an electron configuration of 1s²2s²2p⁶3s²3p⁵.
- The 3p electron we're considering is shielded by:
- 0.35 x 5 (other 3p electrons) = 1.75
- 0.85 x 2 (3s electrons) = 1.70
- 1.00 x 8 (2s and 2p electrons) = 8.00
- 1.00 x 2 (1s electrons) = 2.00
- Total shielding (S) = 1.75 + 1.70 + 8.00 + 2.00 = 13.45
- Z (atomic number of chlorine) = 17
- Z<sub>eff</sub> = 17 - 13.45 = 3.55
More Sophisticated Methods: Quantum Mechanical Calculations
More accurate calculations of Z<sub>eff</sub> require advanced quantum mechanical methods, such as Hartree-Fock calculations or Density Functional Theory (DFT). These methods solve the Schrödinger equation (or variations thereof) for multi-electron atoms, providing a more precise description of electron distribution and, consequently, Z<sub>eff</sub>. These calculations, however, are far more computationally intensive than Slater's rules.
Trends in Effective Nuclear Charge across the Periodic Table
Understanding the trends in Z<sub>eff</sub> across the periodic table is crucial for predicting atomic properties.
-
Across a Period (Left to Right): Z<sub>eff</sub> generally increases across a period. As you move from left to right, the nuclear charge (Z) increases by one with each element, while the shielding effect increases relatively less. This leads to a stronger net positive charge experienced by the valence electrons.
-
Down a Group (Top to Bottom): Z<sub>eff</sub> increases only slightly down a group. While both nuclear charge and the number of shielding electrons increase, the increase in shielding is almost equal to the increase in nuclear charge. The addition of a new electron shell significantly increases the distance of the valence electrons from the nucleus, mitigating the effect of the increased nuclear charge.
Relationship between Effective Nuclear Charge and Atomic Properties
Z<sub>eff</sub> plays a significant role in determining several fundamental atomic properties:
Atomic Radius
As Z<sub>eff</sub> increases, the valence electrons are more strongly attracted to the nucleus, leading to a smaller atomic radius. This explains why atomic radius generally decreases across a period and increases down a group.
Ionization Energy
Ionization energy is the energy required to remove an electron from an atom. A higher Z<sub>eff</sub> results in a higher ionization energy because the valence electrons are more tightly bound to the nucleus. This trend is consistent with the observed increase in ionization energy across a period and decrease down a group.
Electronegativity
Electronegativity is the ability of an atom to attract electrons towards itself in a chemical bond. A higher Z<sub>eff</sub> means that the atom has a stronger pull on electrons, resulting in higher electronegativity. Therefore, electronegativity increases across a period and decreases down a group.
Applications of Effective Nuclear Charge
The concept of Z<sub>eff</sub> has broad applications in various fields of chemistry:
Chemical Bonding
Z<sub>eff</sub> helps in understanding the nature of chemical bonds. Atoms with high Z<sub>eff</sub> tend to form stronger bonds due to the increased attractive forces between the atoms.
Spectroscopic Analysis
Z<sub>eff</sub> influences the energy levels of electrons, which in turn affect the wavelengths of light absorbed or emitted by atoms. This is crucial in spectroscopic techniques used for identifying and analyzing substances.
Predicting Chemical Reactivity
Z<sub>eff</sub> can be used to predict the reactivity of elements. Atoms with low Z<sub>eff</sub> tend to be more reactive as their valence electrons are loosely held.
Material Science
Understanding Z<sub>eff</sub> helps predict the properties of materials, including their conductivity, melting points, and mechanical strength.
Beyond the Basics: Limitations and Refinements
While Z<sub>eff</sub> is a powerful concept, it's essential to acknowledge its limitations:
-
Approximations: Calculations of Z<sub>eff</sub>, especially using Slater's rules, are approximations. More sophisticated methods provide greater accuracy but are computationally demanding.
-
Average Effect: Z<sub>eff</sub> represents an average effect. The actual charge experienced by an electron can fluctuate due to instantaneous electron-electron repulsions.
-
Penetration Effects: The assumption of uniform shielding is a simplification. Electrons in s and p orbitals, which have a higher probability of being close to the nucleus (penetration effect), experience a higher Z<sub>eff</sub> than electrons in d and f orbitals.
Despite these limitations, effective nuclear charge remains an invaluable tool for understanding the fundamental properties of atoms and their behavior in chemical reactions. By considering both the nuclear charge and the shielding effect, Z<sub>eff</sub> provides a crucial link between atomic structure and chemical properties. Further advancements in computational chemistry continue to refine our understanding and calculation of this essential parameter. The deeper we delve into Z<sub>eff</sub>, the clearer the picture becomes of the intricate world of atomic interactions and the resulting properties of matter.
Latest Posts
Latest Posts
-
Atoms Of The Same Element Have The Same Number Of
Mar 23, 2025
-
Individuals Considered Members Of The Same Social Category Or Group
Mar 23, 2025
-
One To One Property Of Logarithms
Mar 23, 2025
-
How Much Atp Is Produced In Etc
Mar 23, 2025
-
The Characteristics Of The Individuals Within The Population
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Effective Nuclear Charge Zeff Is Defined As . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
