Atoms Of The Same Element Have The Same Number Of
Muz Play
Mar 23, 2025 · 5 min read
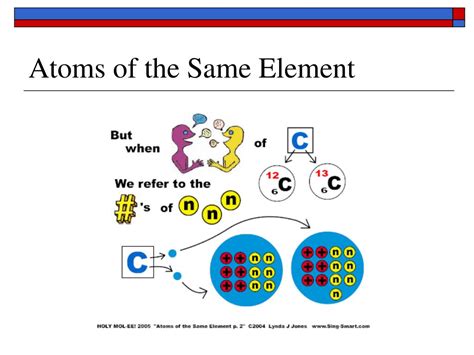
Table of Contents
Atoms of the Same Element Have the Same Number of Protons: A Deep Dive into Atomic Structure
The fundamental building blocks of matter are atoms. While seemingly simple, atoms possess a rich internal structure that dictates their properties and interactions. A crucial aspect of atomic structure, and a cornerstone of chemistry, is the understanding that atoms of the same element have the same number of protons. This seemingly straightforward statement underpins a vast array of scientific concepts and applications. Let's delve into the details, exploring the implications of this fundamental principle.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Atoms consist of three primary subatomic particles:
- Protons: Positively charged particles located within the atom's nucleus.
- Neutrons: Neutral particles (no charge) also residing in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels.
The number of protons an atom possesses defines its atomic number and determines its identity as a specific element. This is the key to understanding the statement "atoms of the same element have the same number of protons." Every hydrogen atom, for example, possesses one proton. Every oxygen atom has eight protons. This unwavering consistency distinguishes one element from another.
Isotopes: Variations in Neutron Number
While the number of protons remains constant for a given element, the number of neutrons can vary. Atoms of the same element with differing neutron numbers are called isotopes. For instance, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C), a radioactive isotope, has six protons and eight neutrons. Although isotopes of the same element exhibit different masses due to varying neutron numbers, their chemical properties remain largely consistent because their electron configuration—determined by the number of protons—is identical.
The Significance of Atomic Number
The atomic number is not merely a label; it's a fundamental property that dictates the element's chemical behavior. The arrangement of electrons in an atom's shells is directly determined by the number of protons. Electrons in the outermost shell, known as valence electrons, participate in chemical bonding. Since atoms of the same element have the same number of protons, they inherently possess the same number of electrons (in a neutral atom) and, therefore, the same valence electron configuration. This identical electron configuration leads to similar chemical reactivity and bonding patterns.
Periodic Table Organization: A Reflection of Atomic Number
The organization of the periodic table perfectly reflects the concept of atomic number. Elements are arranged in order of increasing atomic number, showcasing the periodic recurrence of chemical properties. Elements in the same column (group) possess similar valence electron configurations, resulting in similar chemical behavior. Understanding atomic number and its relationship to electron configuration is essential for predicting and interpreting chemical reactions.
Implications of Identical Proton Numbers
The principle that atoms of the same element have the same number of protons has far-reaching implications across various scientific disciplines:
1. Chemical Reactions and Bonding:
The consistent number of protons determines the element's chemical properties. This is crucial for understanding how elements interact and form chemical bonds. The number of valence electrons, directly tied to the number of protons, dictates the type and number of bonds an atom can form. Without the constancy of proton numbers, predicting chemical reactions would be impossible.
2. Nuclear Chemistry and Radioactivity:
Isotopes, variations in neutron numbers within the same element, play a significant role in nuclear chemistry. While isotopes have different masses, their chemical behavior remains largely consistent because their proton numbers—and thus electron configurations—remain the same. However, differences in neutron numbers can impact nuclear stability, leading to radioactive decay in certain isotopes. Understanding the relationship between proton and neutron numbers is crucial in this field.
3. Spectroscopic Analysis:
Each element emits a unique spectrum of light when energized. This unique spectral fingerprint is directly related to the electron configuration, which is determined by the number of protons. Spectroscopic analysis utilizes this principle to identify the elements present in a sample. The consistency of proton numbers for a given element ensures the reliability of spectroscopic techniques for elemental identification.
4. Mass Spectrometry:
Mass spectrometry is a powerful analytical technique that measures the mass-to-charge ratio of ions. Isotopes of the same element will have different mass-to-charge ratios due to varying neutron numbers, but they will still be identifiable as belonging to the same element based on their characteristic isotopic ratios. The constancy of proton number is implicit in the interpretation of mass spectrometry data.
Exploring Exceptions and Nuances
While the principle is generally true, a few nuanced points are worth considering:
Ions: Charged Atoms
Ions are atoms that have gained or lost electrons, resulting in a net positive (cations) or negative (anions) charge. While the number of protons remains unchanged, the number of electrons differs, affecting the ion's overall charge and properties. However, the identity of the element is still defined by its proton number, not its charge.
Nuclear Reactions: Transmutation
Nuclear reactions can change the number of protons in an atom's nucleus, leading to the formation of a different element—a process known as transmutation. This process fundamentally alters the atom's identity, changing its atomic number and therefore its chemical properties.
Conclusion: The Cornerstone of Chemistry
The principle that atoms of the same element have the same number of protons is a fundamental concept in chemistry and physics. It underpins our understanding of atomic structure, chemical bonding, nuclear chemistry, and analytical techniques. The consistent number of protons determines an element's identity, its chemical behavior, and its unique spectroscopic signature. This fundamental truth serves as a cornerstone upon which a vast body of scientific knowledge is built, impacting various fields from materials science to medicine. The continued exploration of atomic structure and its implications remains a vital area of scientific research, continuously refining our understanding of the universe and its fundamental constituents.
Latest Posts
Latest Posts
-
How Many Electrons Does Tin Have
Mar 24, 2025
-
What Are Functions Of A Family
Mar 24, 2025
-
A Bird Building Their Nest In A Tree
Mar 24, 2025
-
Different Conformations Of The Same Molecule
Mar 24, 2025
-
Does Gas Have A Fixed Shape
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Atoms Of The Same Element Have The Same Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
