How Much Atp Is Produced In Etc
Muz Play
Mar 23, 2025 · 6 min read
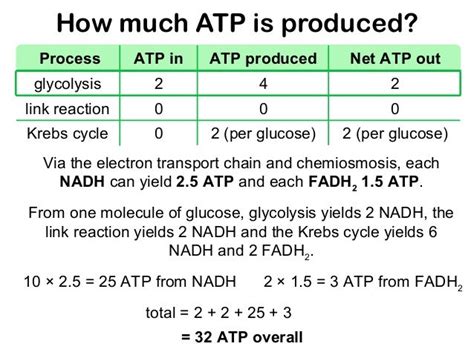
Table of Contents
How Much ATP is Produced in the Electron Transport Chain (ETC)? A Deep Dive into Cellular Respiration
The electron transport chain (ETC), also known as the respiratory chain, is a crucial component of cellular respiration, the process by which cells generate energy in the form of ATP (adenosine triphosphate). Understanding the precise ATP yield of the ETC is complex, and the exact number varies depending on several factors, including the organism, the specific metabolic pathway used, and the efficiency of the process. However, we can break down the process to provide a clear understanding of the theoretical and actual ATP production.
The Electron Transport Chain: A Step-by-Step Overview
Before diving into the ATP yield, let's review the fundamental workings of the ETC. This intricate system is embedded within the inner mitochondrial membrane in eukaryotes and the plasma membrane in prokaryotes. It comprises a series of protein complexes (Complexes I-IV) and mobile electron carriers, namely ubiquinone (Q or CoQ) and cytochrome c. The ETC's primary function is to harness the energy stored in the high-energy electrons derived from the breakdown of glucose during glycolysis and the citric acid cycle (Krebs cycle).
The process unfolds as follows:
-
NADH and FADH2 Delivery: NADH and FADH2, electron carriers generated during glycolysis and the citric acid cycle, deliver their high-energy electrons to the ETC. NADH delivers its electrons to Complex I, while FADH2 delivers its electrons to Complex II.
-
Electron Transfer Through Complexes: The electrons are then passed along a series of redox reactions through Complexes I-IV. Each complex has a progressively higher redox potential, meaning they have an increasingly stronger affinity for electrons. This electron transfer releases energy.
-
Proton Pumping: Crucially, the energy released during electron transfer is used to pump protons (H+) from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space. This creates a proton gradient, also known as a proton motive force (PMF).
-
Oxygen as the Final Electron Acceptor: Finally, at Complex IV, the electrons are transferred to molecular oxygen (O2), which is reduced to water (H2O). Oxygen's high electronegativity makes it an excellent final electron acceptor.
-
Chemiosmosis and ATP Synthesis: The proton gradient established across the inner mitochondrial membrane drives ATP synthesis through chemiosmosis. Protons flow back into the matrix through ATP synthase, an enzyme that harnesses the energy of this proton flow to phosphorylate ADP (adenosine diphosphate) to ATP.
Calculating ATP Yield: Theoretical vs. Actual
The theoretical ATP yield from the ETC is often cited as being around 32-34 ATP molecules per glucose molecule. This calculation is based on the following assumptions:
-
NADH Yield: 10 NADH molecules are produced per glucose molecule during glycolysis and the citric acid cycle. Each NADH molecule theoretically contributes to the pumping of enough protons to generate approximately 2.5 ATP molecules through ATP synthase. This means 10 NADH * 2.5 ATP/NADH = 25 ATP.
-
FADH2 Yield: 2 FADH2 molecules are produced per glucose molecule. Each FADH2 molecule contributes to the pumping of fewer protons than NADH, resulting in approximately 1.5 ATP molecules per FADH2. This equates to 2 FADH2 * 1.5 ATP/FADH2 = 3 ATP.
-
Total Theoretical Yield: Adding the ATP from NADH and FADH2 yields a theoretical maximum of 25 ATP + 3 ATP = 28 ATP from the ETC alone. However, we must also consider the ATP produced during glycolysis (2 ATP) and the citric acid cycle (2 ATP), bringing the total theoretical yield to approximately 32 ATP per glucose molecule.
Why the discrepancy between theoretical (32 ATP) and the often-cited 32-34 ATP? The additional 2-4 ATP often mentioned stems from the fact that the actual efficiency of proton pumping and ATP synthesis is not always perfect. Factors influencing the actual yield include:
-
Proton Leak: Some protons may leak across the inner mitochondrial membrane without passing through ATP synthase, reducing the efficiency of ATP production.
-
Shuttle Systems: The transport of NADH from glycolysis into the mitochondria involves different shuttle systems (e.g., malate-aspartate shuttle, glycerol-3-phosphate shuttle), which have varying efficiencies. The malate-aspartate shuttle is more efficient, yielding 2.5 ATP per NADH, while the glycerol-3-phosphate shuttle yields only 1.5 ATP per NADH.
-
Energy Costs: The transport of metabolites across membranes requires energy, slightly reducing the net ATP gain.
-
Temperature and pH: Variations in temperature and pH can affect the efficiency of enzyme activity within the ETC and ATP synthase.
Factors Affecting ETC Efficiency and ATP Production:
Several factors influence the efficiency of the electron transport chain and the subsequent ATP production. These include:
-
Substrate Availability: The availability of substrates like NADH and FADH2 directly impacts the rate of electron flow and, consequently, ATP production. A limited supply of these molecules will reduce the ATP yield.
-
Oxygen Availability: Oxygen serves as the final electron acceptor. Insufficient oxygen can lead to a build-up of reduced electron carriers and a halt to electron flow, effectively shutting down ATP production. This is evident in conditions of hypoxia or anoxia.
-
Inhibitor Presence: Certain substances can inhibit the activity of specific complexes within the ETC. For example, cyanide inhibits Complex IV, preventing electron transfer and halting ATP production. Rotenone inhibits Complex I, and antimycin A inhibits Complex III. These inhibitors have devastating effects on cellular respiration.
-
Uncoupling Proteins: Uncoupling proteins (UCPs) are located in the inner mitochondrial membrane and facilitate the flow of protons back into the matrix without passing through ATP synthase. This generates heat instead of ATP and is a vital mechanism in thermogenesis (heat generation), particularly in brown adipose tissue.
The Importance of Precise ATP Yield Understanding
Precisely knowing the ATP yield from the ETC is crucial for various aspects of biology and medicine:
-
Understanding Metabolic Disorders: Errors in the ETC can lead to mitochondrial diseases, characterized by impaired ATP production. A deep understanding of ETC function helps in diagnosing and treating these conditions.
-
Drug Development: Many drugs target components of the ETC, such as inhibitors used in cancer therapy or other diseases. Knowing the precise effects of these drugs on ATP production is crucial for optimizing their therapeutic effects and minimizing side effects.
-
Understanding Cellular Energetics: The efficiency of ATP production influences the energy budget of cells, which determines their growth, function, and overall health.
-
Ecological Studies: Understanding the energy efficiency of ETCs in various organisms can provide insights into adaptation and survival strategies in different environments.
Conclusion: A Dynamic and Variable Process
The precise ATP yield from the electron transport chain is not a fixed number but rather a dynamic value influenced by various factors. While the theoretical yield is often estimated around 28 ATP from the ETC (plus additional ATP from glycolysis and the citric acid cycle), the actual yield can vary due to factors such as proton leak, shuttle system efficiency, and environmental conditions. A thorough understanding of these complexities is crucial for appreciating the intricate workings of cellular respiration and its importance in sustaining life. Further research continues to refine our understanding of the intricacies of the electron transport chain and its impact on cellular energy production.
Latest Posts
Latest Posts
-
Is Oxygen A Metal Or Nonmetal Or Metalloid
Mar 24, 2025
-
Label The Indicated Muscles Of The Head And Neck
Mar 24, 2025
-
How To Shift A Graph To The Right
Mar 24, 2025
-
Can You Label The Structures Of A Plant Cell
Mar 24, 2025
-
Is Malleable A Physical Or Chemical Property
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about How Much Atp Is Produced In Etc . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
