Electrolytes Conduct Electrical Currents In Solution
Muz Play
Mar 16, 2025 · 7 min read
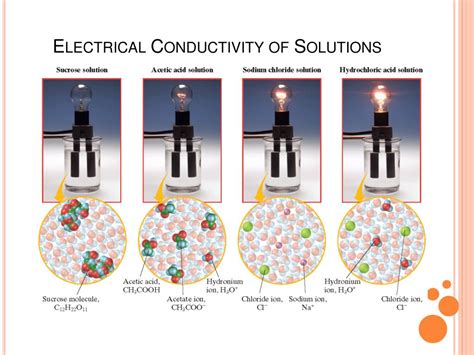
Table of Contents
Electrolytes: The Unsung Heroes of Electrical Conductivity in Solution
Electrolytes are substances that, when dissolved in a solvent like water, produce a solution that can conduct electricity. This seemingly simple phenomenon underpins a vast array of biological processes, industrial applications, and technological advancements. Understanding how electrolytes conduct electrical currents in solution is crucial to grasping their importance across diverse fields. This article delves deep into the mechanisms behind this conductivity, exploring the key players, influencing factors, and the profound implications of this property.
The Role of Ions in Electrical Conductivity
The ability of an electrolyte solution to conduct electricity hinges on the presence of ions. These are electrically charged atoms or molecules formed when an electrolyte dissociates in a solvent. Dissociation is the process where the electrolyte molecule breaks apart into its constituent ions, typically a positively charged cation and a negatively charged anion. It's these mobile ions, free to move within the solution, that facilitate the flow of electrical current.
Strong vs. Weak Electrolytes: A Tale of Two Dissociation
Electrolytes aren't all created equal. Their ability to dissociate and thus conduct electricity varies significantly. We categorize them into two main groups:
-
Strong Electrolytes: These substances completely dissociate into ions when dissolved. Examples include strong acids (like hydrochloric acid, HCl), strong bases (like sodium hydroxide, NaOH), and many salts (like sodium chloride, NaCl). In a solution of a strong electrolyte, virtually all the solute exists as free ions, leading to high electrical conductivity.
-
Weak Electrolytes: These substances only partially dissociate into ions. Acetic acid (CH₃COOH), a component of vinegar, and ammonia (NH₃) are prime examples. In a weak electrolyte solution, only a small fraction of the solute exists as ions, resulting in lower electrical conductivity compared to strong electrolytes. The equilibrium between undissociated molecules and ions dictates the extent of conductivity.
The Mechanism of Conduction: Ion Mobility and Current Flow
When an electric field is applied across an electrolyte solution (e.g., by connecting electrodes to a power source), the mobile ions begin to migrate. Cations, with their positive charge, move towards the negatively charged cathode, while anions, with their negative charge, move towards the positively charged anode. This directed movement of ions constitutes the electrical current.
Several factors influence the efficiency of this ion migration and therefore the overall conductivity:
-
Ion Concentration: Higher ion concentration means more charge carriers available to conduct electricity, leading to higher conductivity.
-
Ion Mobility: Different ions have different sizes and charges, which affect how easily they move through the solution. Smaller ions with higher charges tend to have higher mobility, contributing to greater conductivity. Solvent viscosity also plays a significant role; higher viscosity hinders ion movement and reduces conductivity.
-
Temperature: Increasing temperature generally increases ion mobility by reducing the solvent's viscosity and increasing kinetic energy. This leads to higher conductivity.
-
Solvent Properties: The nature of the solvent strongly impacts electrolyte dissociation and ion mobility. Water, with its high polarity, is an excellent solvent for many ionic compounds, promoting their dissociation and facilitating electrical conductivity. Nonpolar solvents, however, tend to suppress dissociation, leading to lower conductivity.
Applications of Electrolyte Conductivity
The ability of electrolytes to conduct electricity finds a vast range of applications across numerous disciplines:
1. Biological Systems: The Foundation of Life
Electrolyte solutions are essential for life itself. Our bodies rely on electrolytes like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and chloride (Cl⁻) ions for crucial functions:
-
Nerve Impulse Transmission: The propagation of nerve impulses depends on the controlled movement of ions across neuronal cell membranes. Changes in ion concentrations create electrical potentials that trigger nerve signals.
-
Muscle Contraction: Muscle contraction is driven by the interplay of calcium ions and other electrolytes, facilitating the interaction of proteins responsible for muscle movement.
-
Fluid Balance: Electrolytes maintain proper fluid balance within and outside cells, regulating osmotic pressure and preventing dehydration or overhydration.
-
Enzyme Activity: Many enzymes require specific ions as cofactors for optimal activity. Electrolyte balance is crucial for proper enzymatic function and metabolic processes.
Imbalances in electrolyte levels can lead to serious health problems, highlighting the critical role of electrolytes in maintaining bodily functions.
2. Batteries and Fuel Cells: Powering Our World
Electrolytes are the heart of batteries and fuel cells, facilitating the flow of ions between electrodes and enabling energy conversion. Different battery types utilize different electrolytes, tailored to specific energy density, safety, and operational requirements. Lithium-ion batteries, for instance, rely on lithium-containing electrolytes for their high energy storage capacity.
3. Electroplating and Electrolysis: Shaping Metals and Molecules
Electroplating uses electrolyte solutions to deposit a thin layer of metal onto a substrate. Electrolysis, on the other hand, employs an electric current to drive non-spontaneous chemical reactions, such as the decomposition of water into hydrogen and oxygen. Both processes rely on the movement of ions in solution to transfer electrons and facilitate the desired chemical transformations.
4. Industrial Processes: From Metal Refining to Water Treatment
Electrolyte solutions play a critical role in various industrial processes. Electrolytic refining of metals, for instance, uses electricity to purify metals by selectively dissolving and redepositing them. Water treatment often employs electrochemical methods to remove impurities, leveraging the properties of electrolyte solutions.
5. Sensors and Biomedical Devices: Monitoring and Treatment
Electrolyte solutions are fundamental components of many sensors and biomedical devices. Electrochemical sensors, for example, exploit changes in electrolyte conductivity to detect the presence and concentration of specific analytes. Implantable medical devices often utilize electrolyte solutions to ensure proper functionality and biocompatibility.
Factors Affecting Electrolyte Conductivity: A Deeper Dive
While we’ve touched on some key factors, let's explore them in more detail:
1. Temperature's Influence: A Kinetic Perspective
Temperature's impact on conductivity is multifaceted. Higher temperatures boost ion kinetic energy, leading to increased ion mobility and, consequently, higher conductivity. However, the relationship isn't always linear. In some cases, increased temperature might also disrupt the solution's structure, potentially affecting ion mobility in more complex ways.
2. Solvent Effects: Polarity and Viscosity
The solvent's polarity is crucial. Polar solvents, like water, effectively solvate ions, promoting dissociation and improving conductivity. Nonpolar solvents, conversely, hinder dissociation, leading to lower conductivity. Solvent viscosity also plays a significant role. Highly viscous solvents restrict ion mobility, thereby reducing conductivity.
3. Ion Size and Charge: Mobility Matters
Ion size and charge significantly influence mobility. Smaller ions generally navigate the solvent more easily, exhibiting higher mobility. Higher charge also leads to stronger interactions with the electric field, further enhancing mobility. The interplay of size and charge determines the overall contribution of an ion to the solution's conductivity.
4. Concentration Effects: Saturation and Dilution
The concentration of ions directly affects conductivity. Higher concentrations lead to a greater number of charge carriers, boosting conductivity. However, there’s a limit. At extremely high concentrations, ion-ion interactions can hinder mobility, potentially reducing conductivity despite the increased number of ions.
Measuring Electrolyte Conductivity: Techniques and Applications
Measuring the conductivity of electrolyte solutions is crucial in various applications. Several techniques are employed, each with its strengths and limitations:
-
Conductivity Meters: These devices directly measure the conductivity of a solution by applying an electric field and measuring the resulting current. They are widely used for routine measurements in various settings.
-
Conductivity Cells: These specialized cells hold the electrolyte solution and contain electrodes to measure the conductivity. The cell constant, a geometric factor, needs to be considered for accurate measurements.
-
Electrochemical Impedance Spectroscopy (EIS): EIS is a more advanced technique that analyzes the frequency-dependent response of an electrolyte solution to an applied electric field. It provides valuable information about the solution's properties, including conductivity and ion mobility.
Conclusion: The Enduring Importance of Electrolytes
Electrolytes and their ability to conduct electrical currents in solution are fundamental to numerous scientific disciplines and technological advancements. From the biological processes sustaining life to the power sources driving our devices and the industrial processes shaping our world, electrolytes play an indispensable role. Understanding the intricacies of their conductivity is essential for further advancements in various fields, promising exciting possibilities for the future. Further research into electrolyte behavior and novel electrolyte materials continues to unlock new applications and improve existing technologies. The unsung heroes of electrical conductivity remain crucial players in shaping our scientific and technological landscape.
Latest Posts
Latest Posts
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
-
Which Polymer Is Composed Of Amino Acids
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electrolytes Conduct Electrical Currents In Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
