Electromagnetic Waves They Carry Energy Proportional To Their Frequency.
Muz Play
Mar 15, 2025 · 6 min read
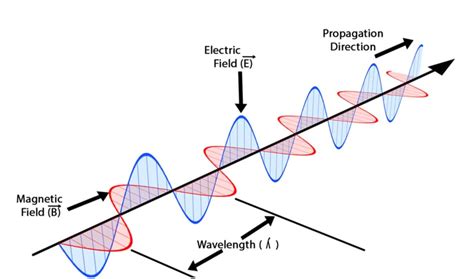
Table of Contents
Electromagnetic Waves: Energy Proportional to Frequency
Electromagnetic (EM) waves are a fundamental aspect of the universe, governing everything from the light we see to the radio waves that power our communication networks. A crucial characteristic of these waves is their energy, which is directly proportional to their frequency. This relationship, described by Planck's equation, underpins many aspects of physics and technology, from the photoelectric effect to the operation of lasers. This article will delve into the nature of electromagnetic waves, exploring their energy-frequency relationship, its implications, and its far-reaching applications.
Understanding Electromagnetic Waves
Electromagnetic waves are disturbances that travel through space by the interplay of oscillating electric and magnetic fields. Unlike mechanical waves, which require a medium to propagate, EM waves can travel through a vacuum, as demonstrated by light from the sun reaching Earth. These waves are transverse, meaning the oscillations of the electric and magnetic fields are perpendicular to the direction of wave propagation.
The Electromagnetic Spectrum
The electromagnetic spectrum encompasses a vast range of frequencies and wavelengths, each with its own distinct properties and applications. This spectrum includes:
- Radio waves: The lowest frequency EM waves, used for communication, broadcasting, and radar.
- Microwaves: Used in cooking, communication, and radar systems. Their shorter wavelengths allow for more precise targeting.
- Infrared (IR) radiation: Experienced as heat, IR radiation is used in thermal imaging, remote controls, and fiber optics.
- Visible light: The portion of the spectrum detectable by the human eye, ranging from violet (highest frequency) to red (lowest frequency).
- Ultraviolet (UV) radiation: Higher frequency than visible light, UV radiation can cause sunburns and is used in sterilization processes.
- X-rays: High-energy EM waves used in medical imaging and material analysis.
- Gamma rays: The highest frequency and most energetic EM waves, originating from radioactive decay and nuclear reactions.
The Energy-Frequency Relationship: Planck's Equation
The cornerstone of understanding the energy of electromagnetic waves is Planck's equation:
E = hf
Where:
- E represents the energy of a photon (a quantum of light).
- h is Planck's constant (approximately 6.626 x 10^-34 Js).
- f is the frequency of the electromagnetic wave.
This equation reveals a fundamental truth: the energy of an electromagnetic wave is directly proportional to its frequency. Higher frequency waves, like gamma rays, carry significantly more energy per photon than lower frequency waves, like radio waves. This relationship is not merely theoretical; it has profound implications across various scientific fields.
Implications of the Energy-Frequency Relationship
The direct proportionality between energy and frequency has numerous implications, including:
1. The Photoelectric Effect
The photoelectric effect, where electrons are emitted from a material when light shines on it, provided crucial experimental evidence for the quantized nature of light. The observation that only light above a certain threshold frequency could eject electrons, regardless of intensity, directly supported Planck's equation. The energy of the incident photons must exceed the work function of the material (the energy required to remove an electron) for the effect to occur. This threshold frequency directly correlates to the minimum energy required for electron emission.
2. Laser Technology
Lasers (Light Amplification by Stimulated Emission of Radiation) rely heavily on the energy-frequency relationship. Lasers produce highly monochromatic (single-frequency) light, meaning all photons possess the same energy. This high energy density allows for precise applications in surgery, material processing, and optical communication. The choice of laser type for a specific application often depends on the desired wavelength and its corresponding energy.
3. Medical Imaging and Treatment
Different EM waves are used in various medical applications based on their energy levels. X-rays, with their high energy, are able to penetrate soft tissues, allowing for imaging of bones and internal organs. Gamma rays are employed in radiation therapy, where their high energy is used to damage cancerous cells. The precise energy levels used are carefully selected to maximize effectiveness while minimizing damage to healthy tissues.
4. Spectroscopy
Spectroscopy involves analyzing the interaction of matter with electromagnetic radiation. By studying the absorption and emission spectra of substances, scientists can identify the elements and molecules present. The specific wavelengths (and thus energies) absorbed or emitted by a substance provide a unique fingerprint, allowing for precise identification and analysis.
5. Astrophysics and Cosmology
The energy-frequency relationship is fundamental in astrophysics and cosmology. Analyzing the electromagnetic radiation emitted by celestial objects, including stars and galaxies, allows scientists to infer their temperature, composition, and motion. Redshift, the stretching of light's wavelength due to the expansion of the universe, is a direct consequence of the change in frequency and hence energy of the photons.
Applications Based on Energy Levels
The versatility of EM waves stems directly from their varying energy levels determined by frequency. Let's explore some specific examples:
High-Energy Applications (UV, X-rays, Gamma Rays):
- Sterilization: UV radiation is effective in killing bacteria and viruses due to its high energy, which damages their DNA.
- Cancer Treatment (Radiation Therapy): Gamma rays' high energy disrupts the DNA of cancer cells, inhibiting their growth and replication.
- Medical Imaging (X-rays): X-rays penetrate soft tissues but are absorbed by denser materials like bones, creating shadow images for medical diagnosis.
- Material Analysis: X-rays and gamma rays are used to analyze the structure and composition of materials through techniques like X-ray diffraction and X-ray fluorescence.
Low-Energy Applications (Radio Waves, Microwaves, Infrared):
- Communication (Radio, TV, Mobile Phones): Radio waves carry information over long distances.
- Heating (Microwaves): Microwaves excite water molecules, causing them to vibrate and generate heat, used in microwave ovens.
- Remote Controls: Infrared radiation is used in remote controls to transmit signals to electronic devices.
- Thermal Imaging: Infrared cameras detect the heat emitted by objects, useful in various applications including security and medical diagnostics.
- Fiber Optics: Infrared light is transmitted through optical fibers for high-speed data communication.
Future Developments and Research
Ongoing research continues to explore the applications and implications of the energy-frequency relationship in EM waves. Areas of active investigation include:
- Advanced Laser Technologies: Developing more powerful and efficient lasers for various applications, including fusion energy and advanced manufacturing.
- Terahertz Technology: Exploring the terahertz frequency range, which falls between microwaves and infrared radiation, for advanced imaging and sensing applications.
- New Medical Imaging Techniques: Developing new medical imaging techniques based on EM waves to improve diagnosis and treatment of diseases.
- Astrophysics and Cosmology: Using advanced telescopes and detectors to observe EM waves from distant galaxies and learn more about the early universe.
Conclusion
The energy-frequency relationship, as described by Planck's equation, is a fundamental principle governing the behavior of electromagnetic waves. This relationship underpins a vast range of technologies and scientific advancements, impacting our daily lives in countless ways. From the light we see to the medical treatments we receive, and from the communication networks that connect us to the exploration of the cosmos, the energy carried by electromagnetic waves, intimately linked to their frequency, remains a crucial aspect of modern science and technology. Further research and innovation in this field promise to continue expanding the possibilities and applications of EM waves for years to come. The seemingly simple equation, E=hf, holds the key to understanding a universe brimming with electromagnetic energy.
Latest Posts
Latest Posts
-
Compare And Contrast Hydrogen Bonds With Van Der Waals Interactions
Mar 15, 2025
-
Magnetic Field At Center Of Loop
Mar 15, 2025
-
Antimicrobial Sensitivity Testing The Kirby Bauer Method
Mar 15, 2025
-
Where Is Feslimc Magma Plate Voundary
Mar 15, 2025
-
T Test For A Single Sample
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Electromagnetic Waves They Carry Energy Proportional To Their Frequency. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
