Electron Configuration For A Neutral Atom Of Phosphorus
Muz Play
Mar 16, 2025 · 6 min read
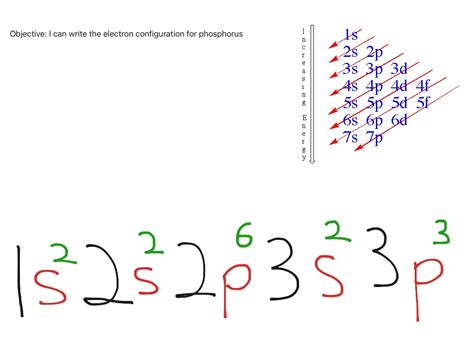
Table of Contents
Electron Configuration for a Neutral Atom of Phosphorus: A Deep Dive
Phosphorus, a fascinating element crucial to life, boasts an intriguing electron configuration. Understanding this configuration unlocks the secrets behind its chemical behavior, reactivity, and place in the periodic table. This comprehensive guide will delve into the electron configuration of a neutral phosphorus atom, explaining the underlying principles and exploring its implications.
Understanding Electron Configuration
Before we dive into the specifics of phosphorus, let's establish a foundational understanding of electron configuration. Electron configuration describes how electrons are arranged in the various energy levels and sublevels within an atom. It follows specific rules dictated by quantum mechanics, including the Aufbau principle, the Pauli exclusion principle, and Hund's rule.
The Aufbau Principle
The Aufbau principle, meaning "building-up" in German, dictates that electrons fill the lowest energy levels first. Think of it like filling a building from the ground floor upwards – you wouldn't start on the tenth floor! Energy levels are represented by principal quantum numbers (n = 1, 2, 3, etc.), with lower 'n' values indicating lower energy levels.
The Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers. This means each orbital (a region of space within a sublevel where an electron is most likely to be found) can hold a maximum of two electrons, and these two electrons must have opposite spins (represented as ↑ and ↓).
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion, leading to a more stable configuration. Imagine assigning seats on a bus – you'll fill each seat individually before putting two people in the same seat.
Determining the Electron Configuration of Phosphorus
Phosphorus (P) has an atomic number of 15, meaning a neutral phosphorus atom contains 15 protons and 15 electrons. To determine its electron configuration, we'll follow the Aufbau principle, filling orbitals in order of increasing energy.
The Order of Filling Orbitals
The order of filling orbitals isn't simply a linear progression of principal quantum numbers. Sublevels within a principal energy level have slightly different energies. The order is generally:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
However, a simplified mnemonic device, like "s, p, d, f, up and down", or diagrams showing the relative energy levels, can be helpful.
Filling the Orbitals for Phosphorus
Now, let's fill the orbitals for phosphorus's 15 electrons:
- 1s²: The first energy level (n=1) contains only one subshell, the 's' subshell, which can hold a maximum of two electrons. We fill it completely.
- 2s²: The second energy level (n=2) starts with the 's' subshell, also holding two electrons. We fill this completely as well.
- 2p⁶: The 'p' subshell within the second energy level can hold up to six electrons (three orbitals, each holding two electrons). We fill this completely.
- 3s²: The third energy level (n=3) begins with the 's' subshell, again holding two electrons. We fill this completely.
- 3p³: Finally, we reach the 'p' subshell in the third energy level. Phosphorus has three remaining electrons, which will individually occupy the three orbitals within the 3p subshell, following Hund's rule.
Therefore, the complete electron configuration for a neutral phosphorus atom is: 1s²2s²2p⁶3s²3p³
Orbital Diagrams and Electron Configuration
Visualizing the electron configuration using orbital diagrams helps clarify the distribution of electrons. Each orbital is represented by a box, and electrons are represented by arrows.
For Phosphorus:
- 1s: ↑↓
- 2s: ↑↓
- 2p: ↑↓ ↑↓ ↑↓
- 3s: ↑↓
- 3p: ↑ ↑ ↑ (Each arrow represents an electron in a separate orbital)
This diagram visually reinforces the application of Hund's rule.
Implications of Phosphorus's Electron Configuration
The electron configuration of phosphorus has profound implications for its chemical properties and reactivity:
Valence Electrons and Reactivity
The valence electrons are the electrons in the outermost energy level, which are involved in chemical bonding. For phosphorus, these are the five electrons in the 3s and 3p subshells (3s²3p³). This means phosphorus has a valence of 5, indicating its tendency to form five covalent bonds or gain three electrons to achieve a stable octet configuration.
Oxidation States
Phosphorus can exhibit various oxidation states, reflecting its ability to lose or gain electrons. Common oxidation states include -3 (gaining three electrons), +3 (losing three electrons), and +5 (losing five electrons). These different oxidation states lead to phosphorus forming a variety of compounds with diverse properties.
Chemical Bonding
The presence of three unpaired electrons in the 3p subshell explains phosphorus's ability to form three single covalent bonds, as seen in molecules like PH₃ (phosphine). The availability of additional orbitals allows for more complex bonding scenarios, such as the formation of PCl₅ (phosphorus pentachloride).
Comparing Phosphorus's Electron Configuration to Other Elements
Comparing phosphorus's electron configuration to elements in the same group (Group 15 or VA) and period (Period 3) highlights periodic trends.
Group 15 (Nitrogen Family)
Nitrogen (N), arsenic (As), antimony (Sb), and bismuth (Bi) all share similar valence electron configurations (ns²np³). This accounts for their similar chemical properties, such as their tendency to form covalent compounds with a valence of 3 or 5. However, as you move down the group, the reactivity generally decreases due to the increasing atomic size and shielding effect.
Period 3 Elements
Comparing phosphorus to other period 3 elements (sodium, magnesium, aluminum, silicon, sulfur, chlorine, and argon) illustrates the relationship between electron configuration and properties. Elements in the same period have the same number of electron shells, but differing numbers of valence electrons. This difference accounts for variations in electronegativity, ionization energy, and reactivity. For example, phosphorus is less electronegative than chlorine and more electronegative than silicon.
Applications and Importance of Phosphorus
Understanding phosphorus's electron configuration is crucial because it explains its essential role in various biological and industrial applications:
-
Biological systems: Phosphorus is a vital component of DNA, RNA, and ATP (adenosine triphosphate), the energy currency of cells. Its unique electron configuration allows for the formation of phosphate bonds, which are crucial for energy storage and transfer in living organisms.
-
Fertilizers: Phosphorus is a key nutrient for plant growth, making it a crucial component of fertilizers. Understanding its chemical behavior and reactivity is essential for developing efficient and sustainable fertilizer technologies.
-
Industrial applications: Phosphorus compounds are used in a variety of industrial applications, including the production of detergents, flame retardants, and pesticides. Its reactivity and ability to form various compounds make it a versatile element in diverse industrial processes.
Conclusion
The electron configuration of a neutral phosphorus atom, 1s²2s²2p⁶3s²3p³, is fundamental to understanding its chemical behavior, reactivity, and applications. By applying the principles of quantum mechanics and the Aufbau principle, Pauli exclusion principle, and Hund's rule, we can accurately predict and explain phosphorus's role in various contexts, from biological systems to industrial processes. This knowledge is crucial for advancements in fields like agriculture, medicine, and materials science, highlighting the importance of comprehending the fundamental principles of electron configuration. Further exploration of phosphorus's chemistry unveils an even richer understanding of this fascinating element's unique properties and significance.
Latest Posts
Latest Posts
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
-
Are Strong Bases Good Leaving Groups
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Electron Configuration For A Neutral Atom Of Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
