Electron Subshells In Order Of Increasing Energy
Muz Play
Mar 20, 2025 · 6 min read
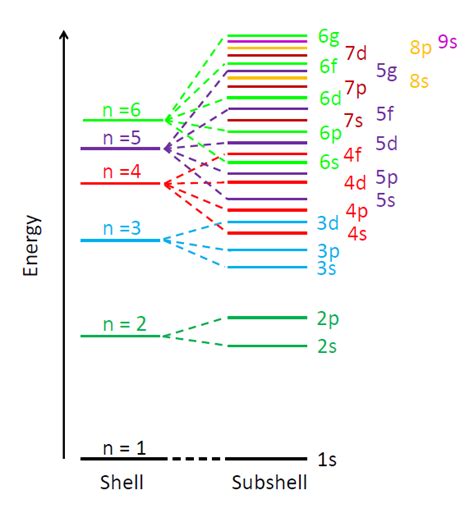
Table of Contents
Electron Subshells in Order of Increasing Energy: A Comprehensive Guide
Understanding the arrangement of electrons within an atom is fundamental to comprehending chemical behavior and properties. This arrangement isn't haphazard; electrons occupy specific energy levels and sublevels, often visualized as shells and subshells. This article delves deep into the order of increasing energy for electron subshells, exploring the underlying principles, exceptions, and practical applications of this crucial concept.
The Quantum Mechanical Model and Electron Configuration
Before diving into the energy order, let's establish a foundation in the quantum mechanical model of the atom. Unlike the simplistic Bohr model, the quantum model describes electrons not as orbiting particles, but as existing in orbitals – regions of space with a high probability of finding an electron. These orbitals are grouped into subshells, and subshells into shells.
-
Shells (Principal Quantum Number, n): These represent the main energy levels, denoted by integers (n = 1, 2, 3, etc.). Higher values of 'n' indicate higher energy levels and greater distance from the nucleus.
-
Subshells (Azimuthal Quantum Number, l): Within each shell, there are subshells with slightly different energies. The azimuthal quantum number, 'l', determines the shape and number of orbitals within a subshell. 'l' can have integer values from 0 to n-1. These subshells are designated by letters:
- l = 0: s subshell (spherical)
- l = 1: p subshell (dumbbell-shaped)
- l = 2: d subshell (more complex shapes)
- l = 3: f subshell (even more complex shapes)
-
Orbitals (Magnetic Quantum Number, ml): Each subshell contains one or more orbitals. The magnetic quantum number, 'ml', specifies the orientation of the orbital in space. 'ml' can have integer values from -l to +l, including 0. Therefore:
- s subshell has 1 orbital
- p subshell has 3 orbitals
- d subshell has 5 orbitals
- f subshell has 7 orbitals
-
Electron Spin (Spin Quantum Number, ms): Each orbital can hold a maximum of two electrons, with opposite spins (+1/2 and -1/2). This is Pauli's Exclusion Principle.
The Aufbau Principle and the Order of Filling Subshells
The Aufbau principle (German for "building-up") dictates that electrons fill the lowest energy levels first before occupying higher energy levels. However, the order of filling isn't simply a straightforward increase in the principal quantum number (n). The relative energies of subshells can vary depending on the atomic number (number of protons).
The (n+l) Rule: A Guiding Principle
A helpful, though not perfectly accurate, rule of thumb for predicting the order of filling is the (n+l) rule. It states that subshells are filled in increasing order of (n+l). If two subshells have the same (n+l) value, the one with the lower 'n' value fills first.
Let's illustrate this:
| Subshell | n | l | (n+l) |
|---|---|---|---|
| 1s | 1 | 0 | 1 |
| 2s | 2 | 0 | 2 |
| 2p | 2 | 1 | 3 |
| 3s | 3 | 0 | 3 |
| 3p | 3 | 1 | 4 |
| 4s | 4 | 0 | 4 |
| 3d | 3 | 2 | 5 |
| 4p | 4 | 1 | 5 |
| 5s | 5 | 0 | 5 |
| 4d | 4 | 2 | 6 |
| 5p | 5 | 1 | 6 |
| 6s | 6 | 0 | 6 |
| 4f | 4 | 3 | 7 |
| 5d | 5 | 2 | 7 |
| 6p | 6 | 1 | 7 |
| 7s | 7 | 0 | 7 |
| 5f | 5 | 3 | 8 |
| 6d | 6 | 2 | 8 |
| 7p | 7 | 1 | 8 |
Based on this table, the predicted order of filling is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
Exceptions to the (n+l) Rule
It's crucial to understand that the (n+l) rule is a guideline, not an absolute law. Several exceptions exist, particularly for transition metals and lanthanides/actinides. These exceptions arise due to complex electron-electron interactions and relativistic effects that are not captured by the simple (n+l) rule. These interactions slightly alter the energy levels of subshells, leading to deviations in the filling order.
For example, Chromium (Cr) and Copper (Cu) are well-known exceptions. Their electron configurations deviate from the predicted order to achieve a more stable, half-filled or fully-filled d subshell.
-
Chromium (Cr): The expected configuration is [Ar] 4s² 3d⁴, but the actual configuration is [Ar] 4s¹ 3d⁵. The half-filled d subshell is energetically more favorable.
-
Copper (Cu): The expected configuration is [Ar] 4s² 3d⁹, but the actual configuration is [Ar] 4s¹ 3d¹⁰. The fully-filled d subshell is energetically more favorable.
Visualizing the Order: The Aufbau Diagram
A visual aid, often used to remember the electron filling order, is the Aufbau diagram (or Aufbau principle diagram). This diagram arranges the subshells in a manner that reflects the approximate energy order. While not perfectly accurate due to the exceptions mentioned, it's a very useful tool.
Practical Applications of Understanding Electron Subshell Order
The knowledge of electron subshell order and electron configuration is crucial in many areas:
-
Predicting Chemical Properties: The arrangement of electrons in the outermost shell (valence electrons) primarily determines an element's chemical reactivity and bonding behavior.
-
Spectroscopy: Understanding electron transitions between subshells is fundamental to interpreting spectroscopic data, which provides information about the structure and composition of matter.
-
Materials Science: The electronic structure of materials dictates their physical and chemical properties, influencing their applications in various technologies. Understanding electron configuration helps design materials with specific characteristics.
-
Nuclear Chemistry: Electron capture and internal conversion, processes involving nuclear transitions, are directly influenced by electron configuration and the availability of orbitals for electron capture.
-
Computational Chemistry: Accurate modeling of molecular properties requires a detailed understanding of the electron configuration of atoms and molecules. Quantum chemical calculations rely heavily on this knowledge.
Advanced Concepts and Further Exploration
This article provides a foundational understanding of electron subshells and their energy order. However, a deeper exploration into advanced topics can provide further insights:
-
Relativistic Effects: At high atomic numbers, relativistic effects significantly influence electron energies and orbital sizes.
-
Electron Correlation: Electron-electron interactions are complex and difficult to model accurately. Advanced computational techniques are needed to fully account for electron correlation.
-
Hund's Rule: This rule states that electrons fill orbitals within a subshell individually before pairing up, maximizing the total spin.
-
Ionization Energies and Electron Affinities: These properties are directly related to the electron configuration and the energy levels of subshells.
Conclusion
The order of increasing energy for electron subshells is a fundamental concept in chemistry and physics. While the (n+l) rule provides a good first approximation, exceptions exist due to complex interactions. Understanding this order, along with the underlying quantum mechanical principles, is essential for predicting the chemical and physical properties of elements and materials. Continued study and exploration of advanced concepts will further deepen this understanding and contribute to advancements in numerous scientific fields.
Latest Posts
Latest Posts
-
What Are The Reactants Of Fermentation
Mar 21, 2025
-
What Is The Elements Of Culture
Mar 21, 2025
-
Can Pure Substances Be Broken Down
Mar 21, 2025
-
Nakatpase Is Primary Or Secondary Active Transport
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Electron Subshells In Order Of Increasing Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
