Nakatpase Is Primary Or Secondary Active Transport
Muz Play
Mar 21, 2025 · 6 min read
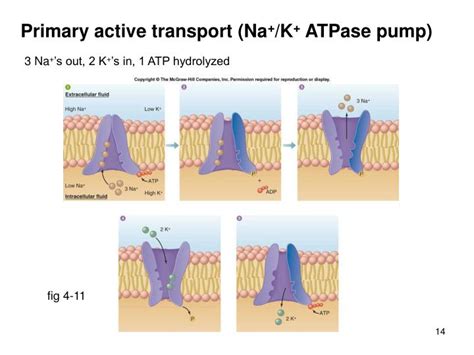
Table of Contents
Is Na+/K+-ATPase Primary or Secondary Active Transport? A Deep Dive into Ion Transport Mechanisms
The sodium-potassium pump, also known as Na+/K+-ATPase, is a crucial transmembrane protein found in virtually all animal cells. Its primary function is to maintain the electrochemical gradient across the cell membrane, a process vital for numerous cellular processes. However, a common point of confusion arises regarding its classification: is Na+/K+-ATPase a primary or secondary active transport protein? The answer, unequivocally, is primary active transport. This article will delve deep into the mechanism of Na+/K+-ATPase, explaining why it's categorized as primary active transport and differentiating it from secondary active transport systems.
Understanding Active Transport
Before delving into the specifics of Na+/K+-ATPase, it's crucial to understand the broader context of active transport. Active transport mechanisms move molecules across cell membranes against their concentration gradient, meaning from an area of low concentration to an area of high concentration. This process requires energy, as it opposes the natural tendency of molecules to diffuse down their concentration gradient. There are two main types of active transport:
-
Primary Active Transport: This type of transport directly utilizes energy from ATP hydrolysis to move molecules against their concentration gradient. The transporter protein itself is an ATPase, meaning it possesses enzymatic activity to hydrolyze ATP.
-
Secondary Active Transport: This type of transport utilizes the energy stored in an electrochemical gradient established by primary active transport. Instead of directly using ATP, it uses the movement of one molecule down its concentration gradient (typically established by a primary active transporter) to drive the movement of another molecule against its gradient. This is often referred to as co-transport or coupled transport.
The Mechanism of Na+/K+-ATPase: A Primary Active Transporter
Na+/K+-ATPase is a classic example of primary active transport. Its mechanism is elegantly intricate and relies directly on the energy derived from ATP hydrolysis. Here's a step-by-step breakdown:
-
Binding of Intracellular Na+: The pump, in its E1 conformation (facing the intracellular space), has high affinity for sodium ions (Na+). Three Na+ ions bind to specific sites within the protein.
-
ATP Hydrolysis: ATP binds to the pump and is subsequently hydrolyzed. This hydrolysis reaction releases energy, causing a conformational change in the protein. This conformational change is crucial, driving the subsequent steps.
-
Phosphorylation and Conformational Change (E1 to E2): The released phosphate group from ATP attaches to the pump, triggering a conformational shift from the E1 to the E2 conformation. This change in shape reduces the pump's affinity for Na+ and increases its affinity for K+.
-
Release of Na+ and Binding of Extracellular K+: The conformational change exposes the Na+ binding sites to the extracellular space, causing the three Na+ ions to be released. Simultaneously, the pump's affinity for potassium ions (K+) increases, and two K+ ions bind to their respective sites.
-
Dephosphorylation and Conformational Change (E2 to E1): The phosphate group detaches from the protein, triggering another conformational change, shifting the pump back to the E1 conformation. This change reduces the affinity for K+ and increases the affinity for Na+.
-
Release of K+: The conformational shift exposes the K+ binding sites to the intracellular space, causing the two K+ ions to be released into the cell. The cycle then repeats.
Crucial Point: Notice that ATP hydrolysis is directly involved in the conformational changes that drive the movement of Na+ and K+ against their concentration gradients. This direct coupling of ATP hydrolysis to ion transport is the hallmark of primary active transport. The pump doesn't rely on a pre-existing gradient established by another transporter; it generates the gradient itself.
Differentiating Primary and Secondary Active Transport
The key difference between primary and secondary active transport lies in the energy source:
| Feature | Primary Active Transport (e.g., Na+/K+-ATPase) | Secondary Active Transport (e.g., Glucose-Na+ Symporter) |
|---|---|---|
| Energy Source | Direct ATP hydrolysis | Electrochemical gradient (often Na+) |
| ATPase Activity | Yes | No |
| Gradient Creation | Creates the gradient | Utilizes a pre-existing gradient |
| Mechanism | Direct coupling of ATP hydrolysis to transport | Coupled transport; one molecule moves down its gradient, driving another against its gradient |
In secondary active transport systems, like the glucose-Na+ symporter, the movement of Na+ down its concentration gradient (established by Na+/K+-ATPase) provides the energy to move glucose against its concentration gradient. The symporter itself doesn't hydrolyze ATP; it merely couples the two transport events.
The Significance of Na+/K+-ATPase as a Primary Active Transporter
The Na+/K+-ATPase plays a multifaceted role in maintaining cellular homeostasis:
-
Maintaining Cell Volume: By regulating the intracellular concentration of ions, it contributes significantly to maintaining cell volume and preventing osmotic lysis or shrinkage.
-
Generating the Membrane Potential: The unequal distribution of Na+ and K+ ions across the membrane creates an electrochemical gradient, which is crucial for nerve impulse transmission, muscle contraction, and other cellular processes. This gradient forms the basis of the resting membrane potential.
-
Driving Secondary Active Transport: As previously mentioned, the Na+ gradient generated by Na+/K+-ATPase powers numerous secondary active transport systems, facilitating the uptake of essential nutrients and the removal of waste products.
-
Regulation of Cellular Processes: The activity of Na+/K+-ATPase is tightly regulated, and its modulation influences various cellular processes, including cell growth, proliferation, and apoptosis.
Addressing Potential Misconceptions
It's important to address potential misconceptions that may lead to confusion about the classification of Na+/K+-ATPase. Some might argue that because it indirectly influences other transport processes (secondary active transport), it shouldn't be solely categorized as a primary active transporter. However, this argument overlooks the core principle: the direct use of ATP hydrolysis to drive ion transport. The indirect effects on other transport systems are a consequence of the primary active transport function of Na+/K+-ATPase, not the defining characteristic of its transport mechanism.
Conclusion
In conclusion, Na+/K+-ATPase is definitively a primary active transporter. Its mechanism directly couples ATP hydrolysis to the movement of Na+ and K+ ions against their concentration gradients. This fundamental process is essential for maintaining cellular homeostasis and powering numerous other cellular processes. Understanding this distinction between primary and secondary active transport is crucial for comprehending the intricate workings of cell membranes and their role in maintaining life. The Na+/K+-ATPase's pivotal role in establishing the electrochemical gradient across cell membranes underpins its critical significance in cellular physiology. Its intricate mechanism, as a primary active transporter, continues to be a subject of intense research and fascination, unveiling further details about its regulatory mechanisms and its contributions to various cellular functions.
Latest Posts
Latest Posts
-
Electric Field Of A Disk Of Charge
Mar 28, 2025
-
Active Transport Must Function Continuously Because
Mar 28, 2025
-
The Basic Functional Unit Of The Kidney Is
Mar 28, 2025
-
Weak Development Of Support Examples In Essay
Mar 28, 2025
-
On The Weak Acid Strong Base Titration Curve
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Nakatpase Is Primary Or Secondary Active Transport . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
