What Are The Reactants Of Fermentation
Muz Play
Mar 21, 2025 · 6 min read
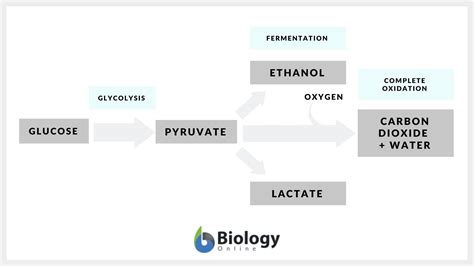
Table of Contents
What Are the Reactants of Fermentation? A Deep Dive into the Process
Fermentation, a metabolic process occurring in the absence of oxygen (anaerobic), plays a crucial role in various biological and industrial applications. Understanding the reactants involved is fundamental to grasping its complexities and harnessing its potential. This article delves deep into the specifics of fermentation reactants, exploring diverse types of fermentation and their unique requirements. We'll uncover the fundamental molecules involved and discuss the influence of environmental factors on the process.
The Core Reactant: A Sugar Source
At the heart of every fermentation process lies a carbohydrate, primarily a sugar, which serves as the primary energy source. This sugar molecule is broken down through a series of enzymatic reactions to yield energy in the form of ATP (adenosine triphosphate) and various byproducts.
While glucose (C₆H₁₂O₆) is often cited as the quintessential fermentation reactant, various other sugars can fuel this process. These include:
1. Glucose: The Universal Fuel
Glucose, a simple sugar, is arguably the most common and versatile substrate for fermentation. Its readily available hydroxyl groups (-OH) facilitate the enzymatic reactions necessary for the process. Many organisms readily metabolize glucose through glycolysis, the central metabolic pathway of fermentation.
2. Fructose: The Fruit Sugar
Fructose, another monosaccharide found abundantly in fruits and honey, is also a readily fermentable sugar. It's isomeric to glucose, meaning it has the same chemical formula but a different structural arrangement. Many microorganisms possess the enzymes necessary to convert fructose into glucose or directly metabolize it through alternative pathways.
3. Sucrose: Table Sugar
Sucrose, or table sugar, is a disaccharide composed of glucose and fructose linked together. Before fermentation can occur, sucrose must be hydrolyzed (broken down) by enzymes like sucrase into its constituent monosaccharides – glucose and fructose – which then serve as substrates for the fermentation process.
4. Lactose: Milk Sugar
Lactose, the principal sugar in milk, is another disaccharide, composed of galactose and glucose. Similar to sucrose, lactose needs to be broken down into its constituent monosaccharides by the enzyme lactase before it can be fermented. This explains why lactose-intolerant individuals experience digestive issues – they lack sufficient lactase to break down lactose effectively.
5. Maltose: Malt Sugar
Maltose, a disaccharide formed from two glucose units, is commonly found in germinated grains (malt). It's readily fermented after being broken down into glucose molecules. The brewing industry heavily relies on maltose fermentation for alcohol production.
6. Starch and Other Polysaccharides: Complex Carbohydrates
Starch, a complex carbohydrate composed of long chains of glucose units, is another important source of fermentable sugars. However, starch needs to be broken down into simpler sugars (primarily glucose) through a process called saccharification, employing enzymes such as amylases. This process is crucial in the production of biofuels from starchy materials like corn and potatoes. Other polysaccharides like cellulose (found in plant cell walls) can also be fermented after suitable pretreatment and enzymatic hydrolysis.
Beyond Sugars: Other Essential Reactants
While sugars form the cornerstone of fermentation, other reactants are also crucial for optimal performance:
1. Water (H₂O): The Universal Solvent
Water acts as the solvent for all the reactions involved in fermentation. It facilitates the transport of reactants, products, and enzymes within the system. Water also participates directly in certain steps of the metabolic pathways.
2. Enzymes: The Biological Catalysts
Fermentation relies heavily on enzymes, which are biological catalysts that accelerate the rate of biochemical reactions without being consumed themselves. These enzymes are specific to the type of fermentation and the sugar being utilized. Examples include:
- Glycolytic enzymes: These enzymes catalyze the breakdown of sugars in glycolysis, the initial phase of fermentation.
- Decarboxylases: These enzymes remove carbon dioxide from intermediate molecules.
- Dehydrogenases: These enzymes transfer electrons (hydrogens) from one molecule to another.
The availability and activity of enzymes are crucial for the efficiency of fermentation. Factors such as temperature, pH, and the presence of inhibitors can significantly affect their function.
3. Nutrients: Supporting Metabolic Processes
Besides the primary sugar source, microorganisms involved in fermentation require other nutrients to thrive. These include:
- Nitrogen sources: These provide the building blocks for amino acids and proteins, essential components of cells. Sources can range from simple ammonia to complex organic nitrogen compounds.
- Phosphorus: Necessary for the synthesis of nucleotides, ATP, and other essential molecules.
- Potassium: Involved in numerous enzyme activities and maintaining osmotic balance.
- Magnesium: A cofactor for several enzymes involved in glycolysis and other metabolic pathways.
- Trace elements: Such as iron, zinc, manganese, and copper, necessary in small quantities for the function of various enzymes.
The precise nutrient requirements vary depending on the microorganism and the specific fermentation process. The absence of essential nutrients can severely limit fermentation efficiency and even lead to its failure.
4. Optimal Environmental Conditions: pH and Temperature
Environmental conditions play a significant role in fermentation. The pH (acidity or alkalinity) of the fermentation medium must be carefully controlled to ensure optimal enzyme activity. Different microorganisms have different pH optima, and the ideal pH varies depending on the specific type of fermentation.
Similarly, temperature significantly impacts enzyme activity. Each enzyme has an optimal temperature range, outside of which activity diminishes. Maintaining the correct temperature is crucial for efficient fermentation.
Different Types of Fermentation and Their Reactants
Various types of fermentation exist, each with its unique metabolic pathways and reactants:
1. Alcoholic Fermentation:
This process, extensively used in brewing and baking, primarily employs glucose or other fermentable sugars as reactants. Yeast, the typical microorganism involved, converts sugars into ethanol (alcohol) and carbon dioxide (CO2).
2. Lactic Acid Fermentation:
This type of fermentation is crucial in the production of yogurt, cheese, sauerkraut, and other fermented foods. Bacteria, such as Lactobacillus and Streptococcus, utilize glucose as the main reactant. The final products are lactic acid and sometimes other minor byproducts.
3. Acetic Acid Fermentation:
This process yields acetic acid (vinegar). Acetic acid bacteria, such as Acetobacter, use ethanol as a reactant, converting it to acetic acid. This implies that acetic acid fermentation often follows alcoholic fermentation.
4. Butyric Acid Fermentation:
This fermentation is less common and is carried out by bacteria such as Clostridium. It typically uses sugars as reactants, resulting in the production of butyric acid, a short-chain fatty acid with a characteristic rancid odor. This process is important in certain industrial applications and also contributes to food spoilage.
5. Propionic Acid Fermentation:
This fermentation, often associated with cheese production, involves bacteria such as Propionibacterium. They utilize lactic acid as a reactant, converting it into propionic acid, acetic acid, and carbon dioxide. This contributes to the characteristic flavor and texture of some cheeses.
Conclusion: A Complex and Versatile Process
Fermentation, a multifaceted anaerobic process, relies on a specific set of reactants to proceed effectively. A fermentable sugar source is the central component, but water, enzymes, and essential nutrients are equally crucial for optimal performance. Various types of fermentation exist, each characterized by specific metabolic pathways and end products. Understanding the reactants and the intricacies of fermentation is vital in various fields, from food production and beverage manufacturing to biofuel production and even waste management. The ongoing research into fermentation continues to unlock its vast potential, promising innovations in various sectors.
Latest Posts
Latest Posts
-
What Marked The End Of The Precambrian Period
Mar 28, 2025
-
What Is A Waste Product Of The Krebs Cycle
Mar 28, 2025
-
North Africa And Southwest Asia Political Map
Mar 28, 2025
-
Periodic Table Of Elements With Ionization Energy
Mar 28, 2025
-
Rate Law For Iodine Clock Reaction
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Are The Reactants Of Fermentation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
